the
TEAM
Meet the visionaries behind Neuro-Kinesis’ unique product platform.
the
TECH
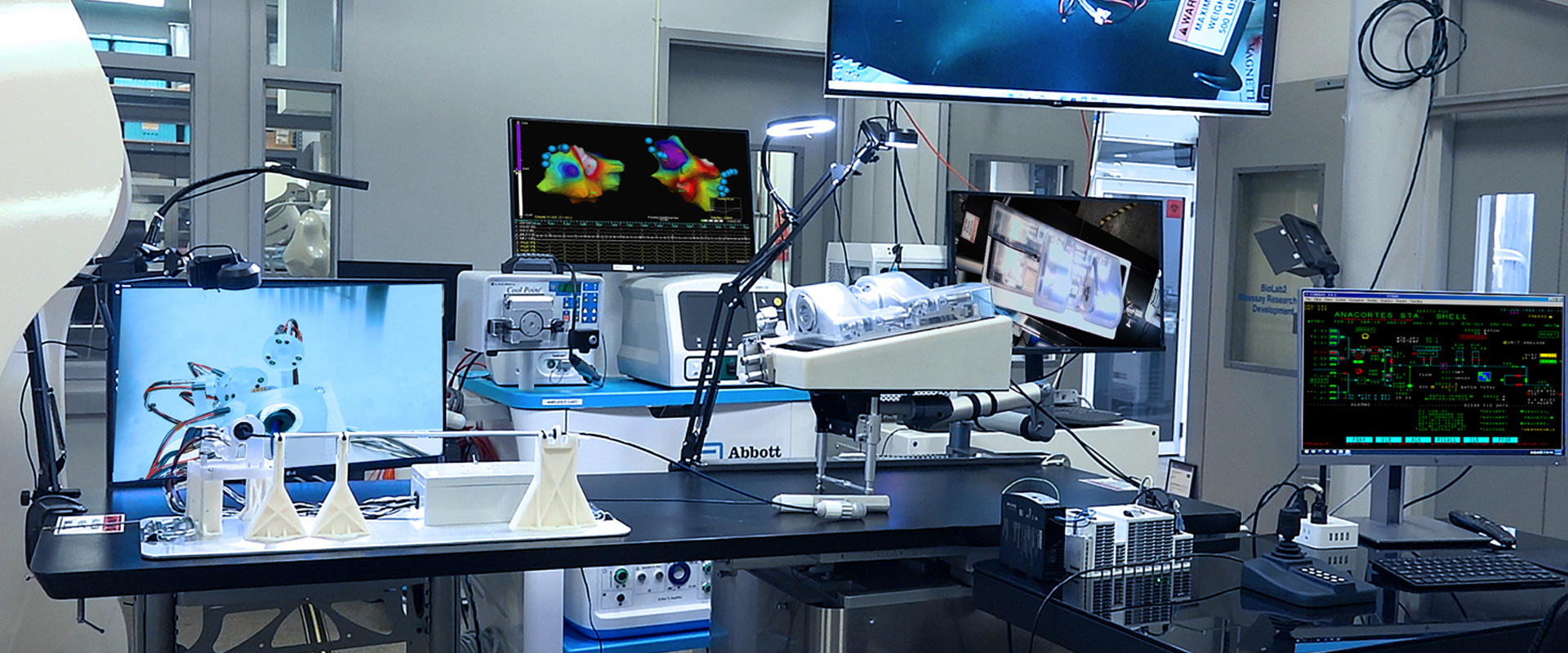
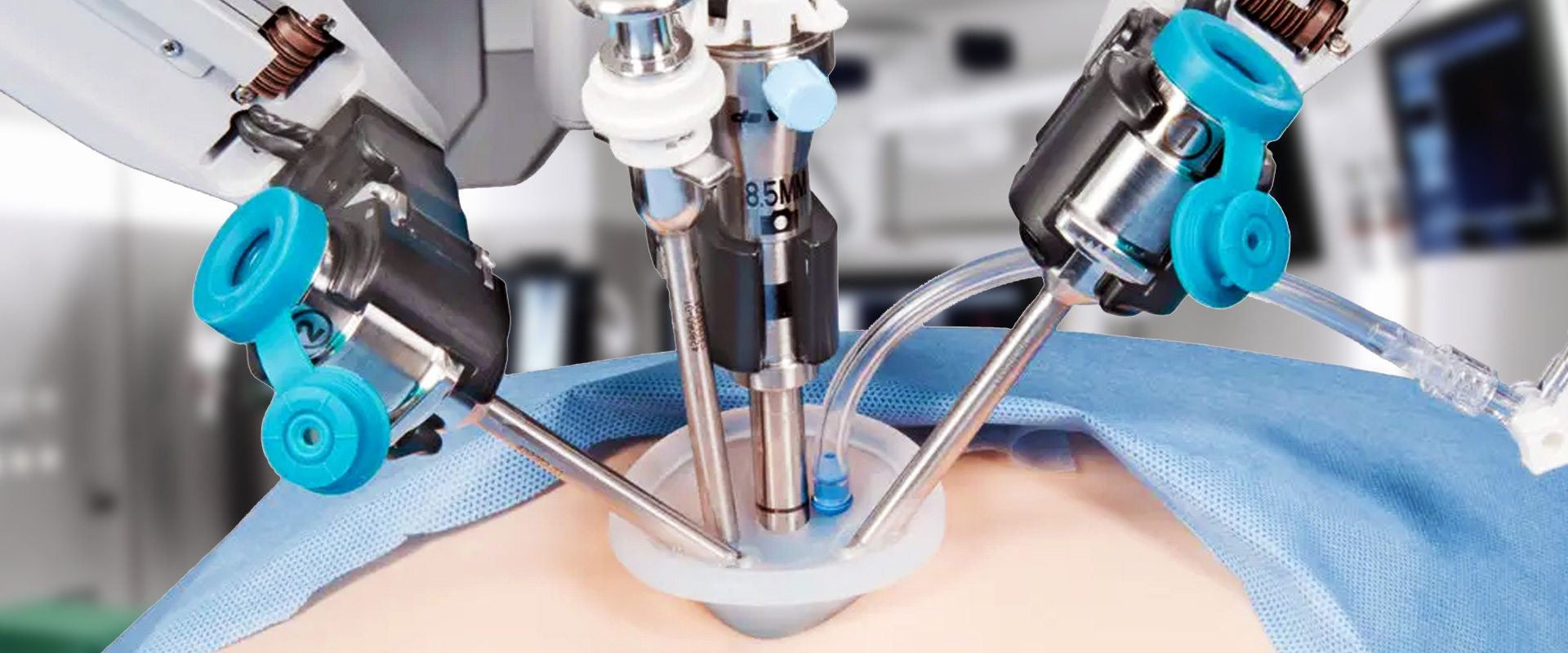
Explore Neuro-Kinesis’ advanced SMART Surgical Tool Technologies

the
TARGET
See how Neuro-Kinesis’ technology will enhance patient outcomes.
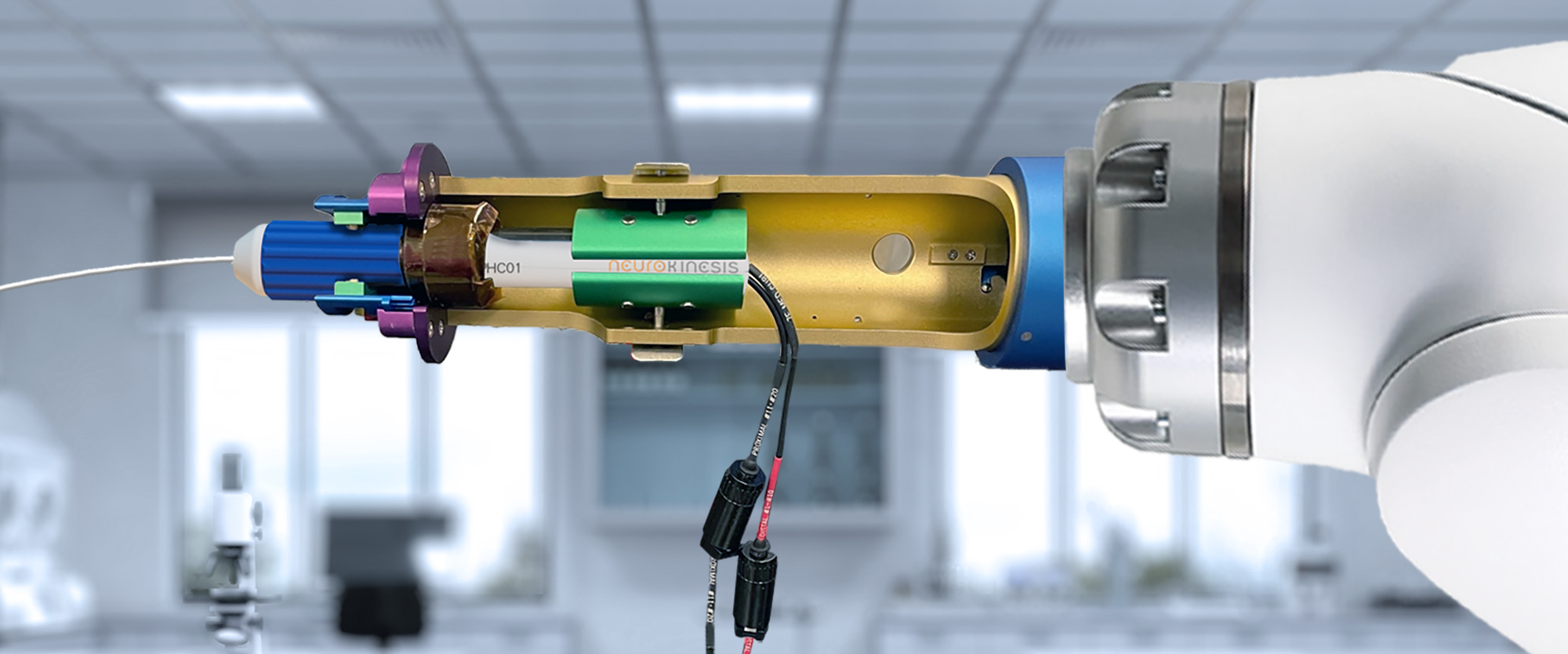
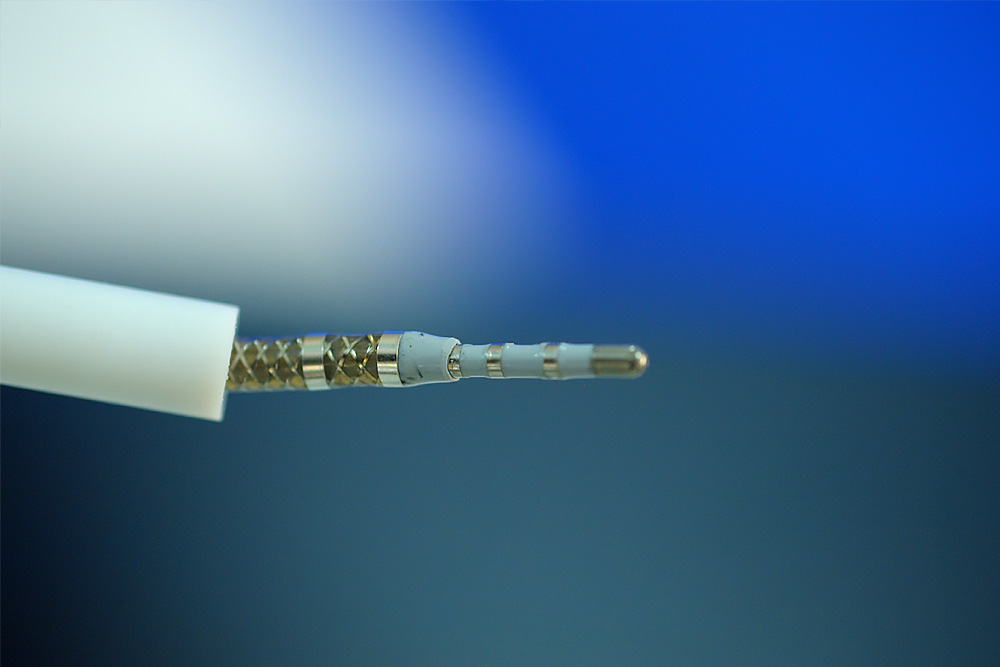
Neuro-Kinesis is focused on creating next-generation surgical tools that incorporate our advanced biosensor technology that provides physicians and surgeons with real-time environmental monitoring that can greatly enhance a patient’s surgical outcome.
NKC Announces Intent To Negotiate Technology Sale to BioSig Technologies
NKC has announced it is negotiation with BioSig Technologies for the sale of some of its IP.
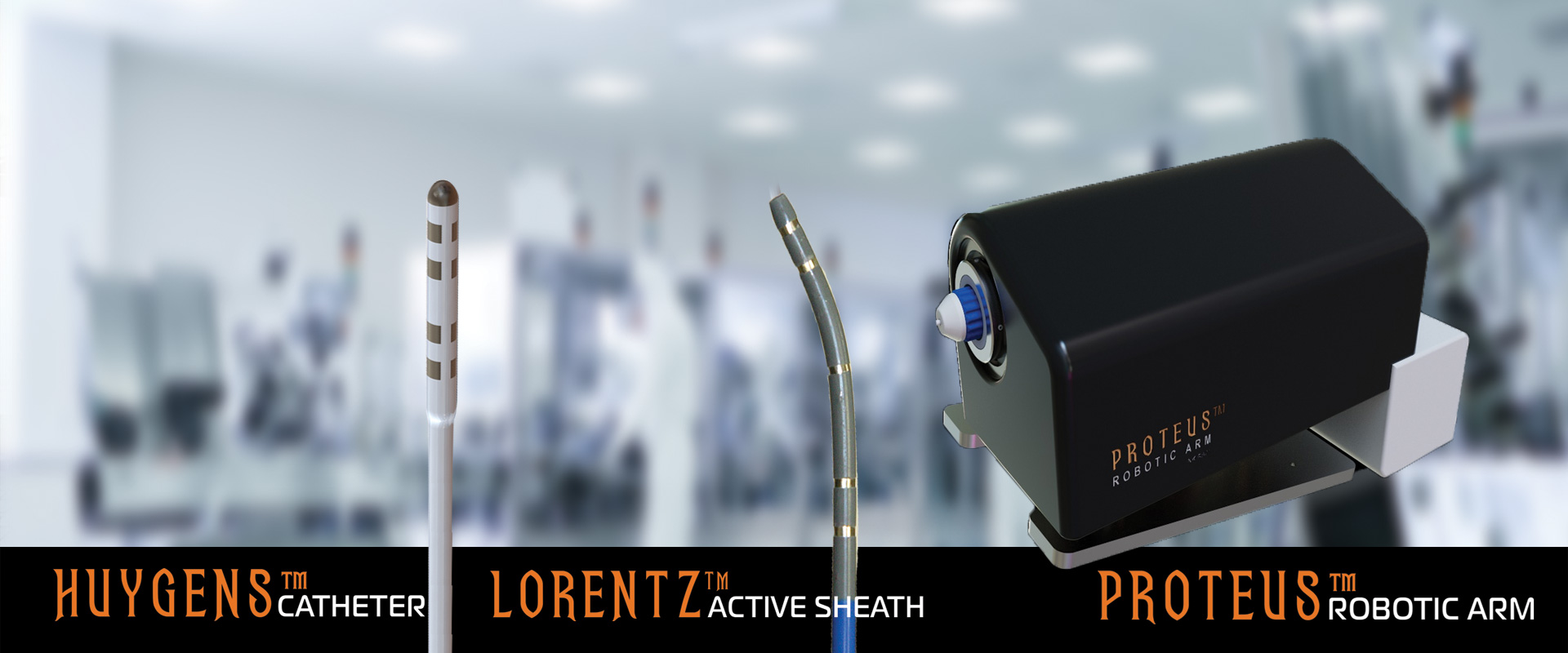
2024 Milestone Progress Update on the Huygens™/ Proteus™ Technology
NKC has released a Milestone Progress Update on the Huygens™/ Proteus™ Technology
2023 End of Year Development Report
NKC has released its End-of-Year Development Report for 2023.
Third Quarter 2023 Development Report
NKC has released its Third Quarter Development Report for 2023.
2022 End-Of-Year Development Report
NKC has released its End-of-Year Development Report for 2022.
Fourth Quarter 2022 Development Report
NKC has released its fourth quarter report for 2022.
Third Quarter 2022 Development Report Released
NKC has released its third quarter development report for 2022.
Abbott Completes The Acquisition Of St. Jude Medical
NKC reports on the acquisition St. Jude Medical by Abbott.
NKC Announces Upcoming Animal Study for Clinical Validation of its Huygens™ – Proteus™ Robotic Arm Surgical Platform
NKC announces animal study to be performed to provide clinical validation of its Huygens™ technology.
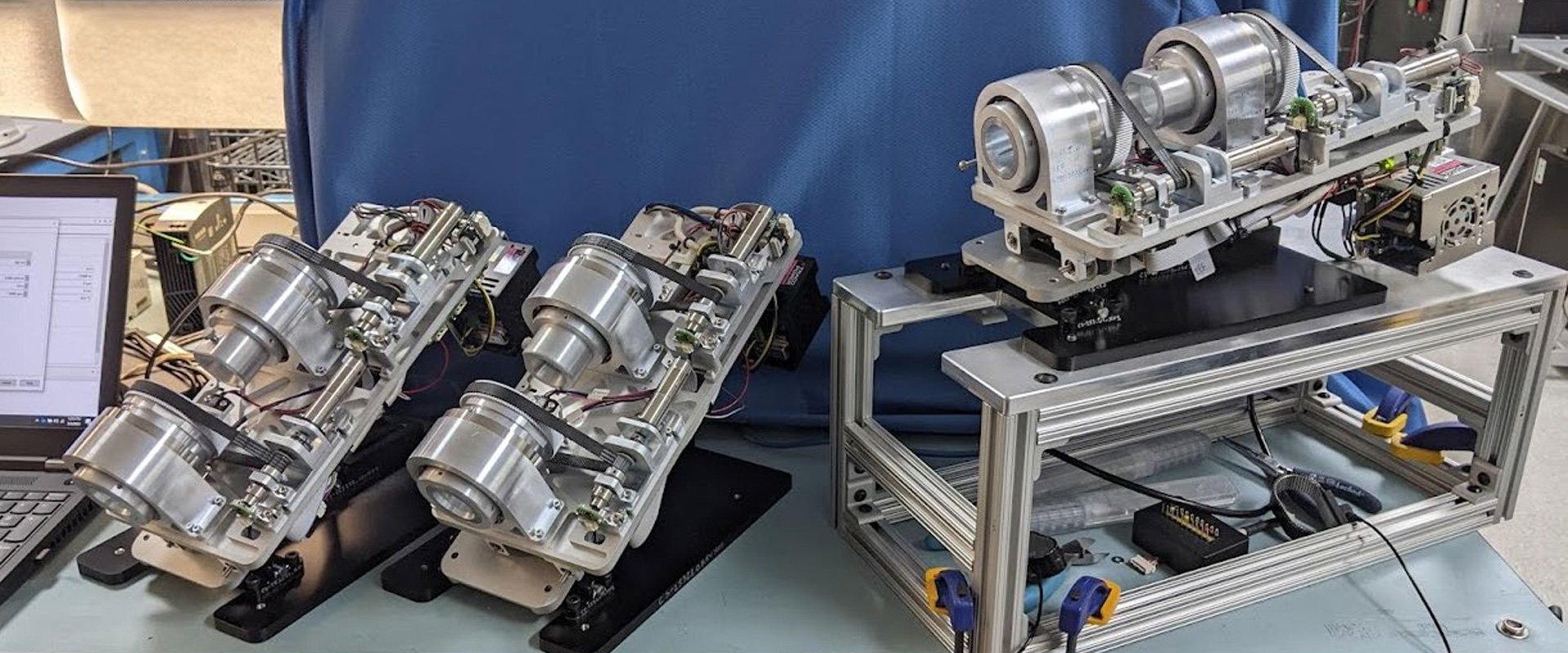
Phase One Development Completed
NKC has announced the completion of the Phase One development for its new Huygens™ Smart Catheter and Proteus™ Robotic Arm Platform.
Frost & Sullivan Report on SBR Market
Global consulting firm Frost & Sullivan looks at potential market impact of the Neuro-Kinesis SmartBrain Retractor technology.
The SMART Tool Revolution
The development of SMART technologies for surgical tools coupled with advances in robotics is providing a revolution in creating better, safer and less invasive surgical options for patients.
Catheter Market Continues to Grow
The global catheter market expected to rise to $77.7B by 2026 with an average CAGR of 9.7% over the forecast period.
CGCI GLP Study Completed
The CGCI platform has successfully undergone a Good Laboratory Practice (GLP) animal study in advance of its next stage clinical trials.