FOR IMMEDIATE RELEASE
NKC Release Fourth Quarter Development Report on the Huygens™ – Proteus™ Robotic Arm Surgical Platform
DATELINE: November 10, 2022, Los Angeles, CA
Neuro-KInesis, Corp. (NKC), is providing the following press release to update its shareholders and strategic partners on the advances made to date in Q422 and to confirm its progress in meeting the milestones set as the company continues to advance the commercialization of its advanced Huygens™ electrophysiology catheter mapping technology and its Proteus™ Robotic-Arm catheter guidance system.
State of the Company

NKC Chairman and CTO Josh Shachar
In a recent conversation with NKC Chairman and CTO Josh Shachar, a clear picture of the current state of advancement with NKC was discussed. Despite several factors in the business sector which continue to pose substantive limitations for those companies in the advanced technology fields, factors such as supply chain issues, human asset acquisition, inflationary pricing, and shifting political climates, NKC has been able to achieve most all of its stated milestones for this year.
“Despite the constraints imposed by the market, NKC has been able to achieve most of its milestones set for this year.“ stated Mr. Shachar, “We have been able to shore up our supply chain issues and grow both our direct engineering staff as well as our strategic development partners in order to keep our momentum moving. Specifically we have been able to sure inventory for the creation of 200 units of our Huygens™ Catheter and our Lorentz™ Active Sheath. We have finalized our partnerships with several key players including Sandia National Laboratories, to handle our catheter validation study, Abbott/St. Jude to integrate their Ensite NavX™ system into the NKC Operating Suite, and we are finalizing the scope of work parameters with Anacaz for developing our AI and cloud-based functionality for our technology platform.
In addition, we have been able to maintain a very streamlined administrative and management operating system that has allowed us to make sure our investor funds is being fiscally focused on only those target areas that move our development to achieve those milestones.
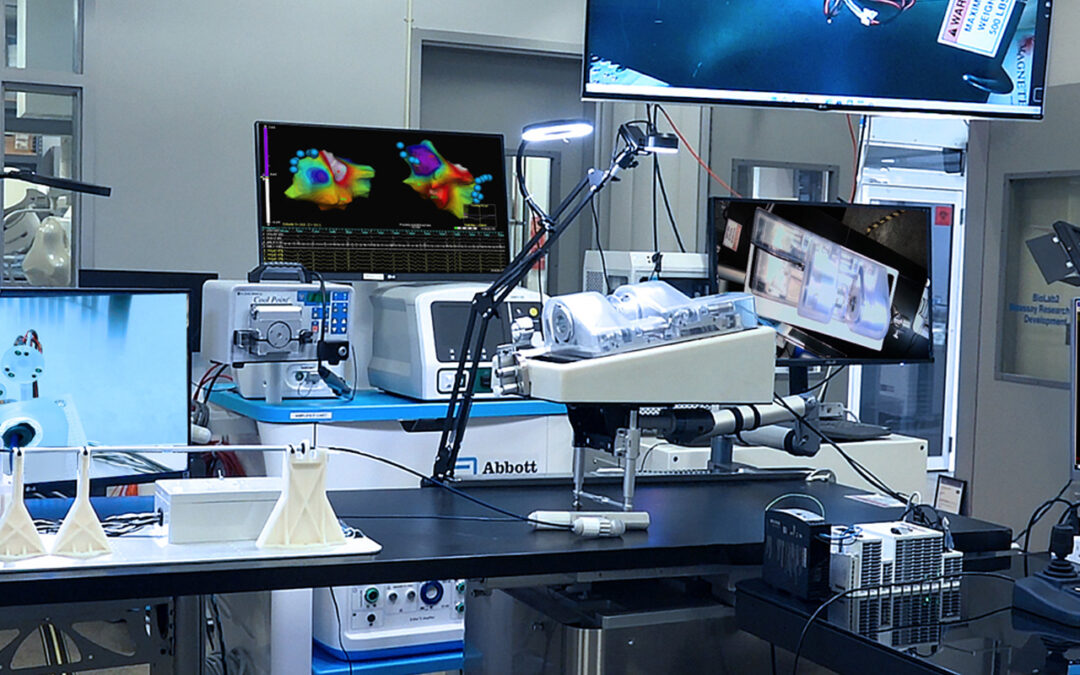
As a result, we have been able to achieve our targeted goals this year including the completion of the Proteus™ Robotic Arm and the IPC communication interface. We have also completed the design and development of the Huygens™ Catheter. In advance of both the animal and Sandia studies, our regulatory team is preparing our submissions to the FDA, and we feel we have established the best pathway to moving through this all-important process as quickly as possible.
“All of this has only been possible because of the commitment of our investor family who continue to see the value in our vision. I am looking forward to our upcoming Shareholder Meeting where we will have the opportunity to share all the achievements we have made and the goals we have set for the upcoming year.
“I remain very confident about the future of NKC. At every step we have made on this path we have seen not only the validation that our technology can do what we envisioned, but in the case of our Huygens™ Catheter technology, we have created a technology that reinvents what we thought was possible in the EP Mapping arena.

NKC ISO 7 Clean Room

Ensite NavX System at NKC Lab
Animal Lab Installation Completed At NKC Headquarters

Proteus™ Robotic Arm at NKC Lab
Initial installation of the prototype mechanical systems developed by Cirtex are already underway as the company moves into the next phase of integrating the AI and robotic automation systems into the technology platform.
Huygens™ Validation Studies on Track To Commence At Sandia National Laboratories.

Sandia’s Dr. Darren Branch to head validation study for Huygens™ Catheter.
As discussed previously, NKC been in discussion with Sandia National Laboratories (Sandia) to perform initial validation studies of its Huygens™ Catheter technology. An agreement has been finalized and the study is anticipated to begin during Q123.
Sandia’s efforts will be to facilitate simulation and analysis of the signal acquisition capabilities of the Huygens™ Catheter’s locally-amplified electrophysiology catheter to validate its advantage over the existing art of EP catheter mapping. The study will be conducted to show whether or not the Huygens™ Catheter is able to effectively capture and measure small microvolt signals such as those that occur in an EP patient whose complex arrhythmias are caused by low-voltage scar tissues. Although current EP mapping catheter technology is capable of identifying high voltage signals, those that lie in a range greater than 500µV (microvolts), they are incapable of accurately reading small microvolt signal such as those that occur in a range as low as 5µV which are indicative of small scar tissue anomalies which can be a primary underlying factor in those complex arrhythmia cases where these electrical disturbances cause irregular pacing issues. If validated by this study, the Huygens™ Catheter will be the first mapping catheter technology to be able to provide the EP physician the ability to accurately map these anomalies. Such an advance would open the door to a whole new level of cure for their patients.

The image above shows the Huygens™ Catheter tabletop circuit board to be used by Sandia for its signal capture certification studies.
Sandia is one of only three advanced research and development laboratories governed by the U.S. National Nuclear Security Administration (NNSA). Though its primary mission remains to maintain the reliability and surety of nuclear weapon systems, conduct research and development in arms control and nonproliferation technologies, Sandia is recognized as one of the nation’s most trusted facilities for the study and certification of technologies and processes related to computational biology, mathematics, and materials science applications. Validation certification from Sandia is considered to be one of the gold-standard pillars in gaining regulatory license from governing bodies such as the FDA in order to move onto human clinical trials and ultimately gaining green-light approval for commercialization.
The image above shows the actual complete FCB that is installed in the tip of the Huygens™ Catheter
The study will be conducted under the direction of Dr. Darren W. Branch, PhD at Sandia’s National Technology & Engineering Solutions Division. Dr. Branch is one of Sandia’s top research scientist specializing in micro and nano systems for chemical and biological analysis as well as bio-sensing, neurological interfaces, and adaptive biological interfaces. Dr. Branch had previously been involved in the development and validation of the pathogen detection system developed by NKC’s sister company, Sensor-Kinesis, a technology that was purchased by Oracle founder Larry Ellison under his AMDI organization, and which is now in the process of being being commercialized internationally.
The validation study protocol will be to take the NKC Huygens™ Catheter and pair it with both an industry standard quadripolar and decapolar mapping catheter to test their ability to measure a variety of electrical and noise signals to establish a comparative dataset to prove the Huygens™ signal capture capabilities in comparison to existing art. Both catheters will be calibrated to the U.S. Primary Standard of 1 microvolt which will guarantee that any reading subsequently measured by the catheters will have no clinically significant error variation between them. Once calibrated, both catheters will be subjected to a variety of tests to analyze their ability to read a variety of signal shapes (sine, sawtooth, triangle, etc) at various voltage levels ranging from 5µV to 80,000 µV. These tests will be performed in both an anechoic chamber, which will provide readings not distorted by any reflection of ambient noise, as well as then introducing signal noise at various frequencies to simulate what is typically encountered in an operating space and which can dramatically pollute endocardial tissue signal measurement.
The results of all these tests will provide the foundational data needed by the FDA for regulatory approval. Of significant interest to NKC in this study will be the proof that the Huygens™ Catheter can not only “see” low-voltage signals, less than 100 µV, but can do so with a resolution and clarity not distorted by any contaminating noise. If shown, this data will provide significant and potentially convincing weight to its claim that it can deliver a reliable ability to measure clinically relevant diagnostic data that was heretofore unavailable which may address a disease model for which there is currently no such diagnostic device.
NKC Will Enter Into Discussions with the FDA for Its Regulatory Approval Process.
It was announced in our Q322 Press Release that both Prof. Elaine Duncan (Paladin Medical Inc.) and NKC consultant Dr. Jaap Laufer MD (Emergo Group Inc. had joined with NKC’s Lead Regulatory Consultant, Susan Alpert MD, to establish the path forward for FDA regulatory approval of the Huygens™ Catheter and the Proteus™ Robotic Arm technology platforms.
The contract regarding the upcoming animal trials to be held at the Technion Institute in Israel has been completed. NKC is developing a Q-Submission for the FDA detailing the current protocol standards that have been outlined for the test. Review and comment by the FDA on this pre-submission will provide NKC with important initial feedback to see if any of its outlined protocols need to be modified to make sure the data requirements the FDA will be looking for have been covered when NKC files for its device certification application.
developing a Q-Submission for the FDA detailing the current protocol standards that have been outlined for the test. Review and comment by the FDA on this pre-submission will provide NKC with important initial feedback to see if any of its outlined protocols need to be modified to make sure the data requirements the FDA will be looking for have been covered when NKC files for its device certification application.
To review, the animal study is being conducted to establish two claims. The first is that the Huygens™ Catheter is able to accurately measure bioelectric conductivity as well as tissue impedance in the dynamic environment of the heart, and two, that it can do so without imposing any safety issues on the subject. This latter point is the more important data the animal trial will provide to help further NKC’s regulatory approval. Device safety from both a mechanical and electrical device standpoint will be important for gaining FDA approval device authorization.
Anticipating positive outcomes from both the Sandia and the Technion studies, NKC is planning on submitting its filing to the FDA for a Humanitarian Use Device (HUD) classification for its Huygens™ Catheter technology. The HUD classification can allow a Class III device manufacturer a much shorter path to in-human clinical trial use than would happen with a standard PMA path. To qualify, the device manufacturer must show two things. The first is that there exists a small population of patients who have a need that is not being met via any existing process or device; and that the development of such a process or device for treating these patients is impossible, highly impracticable, or unsafe. The second is that the manufacturer’s process or device can provide at minimum, a rate of improvement or cure that meets or exceeds current treatment standards, and that their device or process is safe to use and does not create any unknown secondary issues. If this can be demonstrated, then the FDA may grant the HUD certification along with a Humanitarian Device Exemption (HDE). With the HDE, the manufacturer is allowed to make strategic alignments with hospital Institutional Review Boards (IRBs) to begin in-human clinical trials for the patients identified by the HUD. Such trials are limited in scope and the manufacturer, although allowed to recover costs, is not allowed to make a profit during this period.
 If these trials show benefits to the patient and the IRBs, these trials can be expanded. Success from these trials allows the manufacturer to begin to build its case for full PMA approval which will allow the device use to be expanded into broader patient categories and will allow the company to begin to commercialize its invention.
If these trials show benefits to the patient and the IRBs, these trials can be expanded. Success from these trials allows the manufacturer to begin to build its case for full PMA approval which will allow the device use to be expanded into broader patient categories and will allow the company to begin to commercialize its invention.
Currently, NKC’s Chief Medical Officer Dr. Eli Gang is working to gather the documentation needed to show that an under-served patient base exists for the Huygens™ Catheter. NKC has identified that there are a significant number of EP patients whose heart issues have not and cannot be addressed because their disease is the result of complex arrhythmias caused by low-voltage scar tissue. Since current mapping catheter technologies cannot effectively measure the signals associated with this disease model, and as NKC is hoping to prove that its Huygens™ technology can provide an effective solution for this issue, it feels that it is in a strong position to make its case to the FDA for the HUD classification.
In addressing the regulatory process for the Proteus™ Robotic Arm, NKC is currently evaluating the existing robotic catheter guidance systems being marketed today to determine if there are enough similarities to allow the company to file for a 510(k) designation. If the company can show that an existing approved device is in greater part similar to the Proteus™, then that device can be used as a predicate that may allow the FDA to grant a Substantial Equivalence (SE) order. The SE determination will allow NKC to begin marketing the Proteus™ as a Class II Medical Device. If in the evaluation of the Proteus™ technology to existing art it is found to be too unique in nature, or that because its use as part of a larger system may change its classification, then the FDA may require a different regulatory pathway, up to and including a PMA.
NKC Defines Scope of Work With Anacaz
As previously announced, NKC is in negotiations with the Anacaz Group to support the development of the both the firmware and software as well as the cloud-based operations for the Huygens™/Proteus™ technologies and the NKC Operating Suite. Currently, meetings are scheduled between the NKC and the Anacaz Group engineering teams to refine the scope of work required and the timelines to develop test and implement the various programming needs.

Anacaz Group Founder and CEO Rob Abrams
The Anacaz Group is also providing future integration of a cradle-to-grave cloud-based system for the Huygens™ Catheter. Such a system will provide a host of functionality including:
- Unique ID marking of a catheter at its time of manufacture
- Initial calibration ratings for quality control and pre-use comparison.
- Shipping and tracking of the catheter for use and inventory control.
- Pre-procedure calibration checking.
- Remote recalibration if needed.
- Data-capture and storage of the patient’s mapping/ablation procedure into a secure database for physician/patient recall and for comparative study for ongoing technology refinements.
- Final catheter termination disposal information.
All of this will be integrated into a secure network with both Bluetooth and Wi-Fi connectivity to allow remote access to specific technician, administrative, physician and patient portals.
SAFE HARBOR DECLARATION
THE STATEMENTS, PROJECTIONS AND ESTIMATES OF FUTURE PERFORMANCE OF THE COMPANY OR VARIOUS ELEMENTS OF THE COMPANY’S BUSINESS CONTAINED IN THIS MEMORANDUM THAT ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD EXPECT THAT ANTICIPATED EVENTS AND CIRCUMSTANCES MAY NOT OCCUR, THAT UNANTICIPATED EVENTS AND CIRCUMSTANCES WILL OCCUR, AND THAT ACTUAL RESULTS WILL LIKELY VARY FROM THE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD BE AWARE THAT A NUMBER OF FACTORS COULD CAUSE THE FORWARD-LOOKING STATEMENTS OR PROJECTIONS CONTAINED IN THIS MEMORANDUM OR OTHERWISE MADE BY OR ON BEHALF OF THE COMPANY TO BE INCORRECT OR TO DIFFER MATERIALLY FROM ACTUAL RESULTS. SUCH FACTORS MAY INCLUDE, WITHOUT LIMITATION, (i) THE ABILITY OF THE COMPANY TO COMPLETE THE DEVELOPMENT OF ITS PRODUCTS IN A TIMELY MANNER, (ii) THE DEMAND FOR AND TIMING OF DEMAND FOR SUCH PRODUCTS, (iii) COMPETITION FROM OTHER PRODUCTS AND COMPANIES, (iv) THE RESULTS OF THE COMPANY’S SAFETY AND EFFICACY STUDIES, (v) THE RESULTS OF THE REGULATORY APPROVAL PROCESS, (vi) THE COMPANY’S SALES AND MARKETING CAPABILITIES, (vii) THE COMPANY’S ABILITY TO SELL ITS PRODUCTS PROFITABLY, (viii) THE ABILITY OF THE COMPANY’S THIRD-PARTY SUPPLIERS TO PROVIDE PRODUCTS AND SERVICES IN A RELIABLE MANNER; (ix) AVAILABILITY OF ADEQUATE DEBT AND EQUITY FINANCING, AND (x) GENERAL BUSINESS AND ECONOMIC CONDITIONS. THESE IMPORTANT FACTORS AND CERTAIN OTHER FACTORS THAT MIGHT AFFECT THE COMPANY’S FINANCIAL AND BUSINESS RESULTS ARE DISCUSSED IN THIS MEMORANDUM UNDER “RISK FACTORS.” THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL BE ABLE TO ANTICIPATE, RESPOND TO OR ADAPT TO CHANGES IN ANY FACTORS AFFECTING THE COMPANY’S BUSINESS AND FINANCIAL RESULTS.