FOR IMMEDIATE RELEASE
NKC Releases Its End-Of-Year Development Report on the Huygens™/ Proteus™ Technology
DATELINE: December 20, 2022, Los Angeles, CA

CEO Josh Shachar examining the Huygens™ Catheter.
Los Angeles, CA December 20, 2022 – The following is being provided to share the end-of-year updates on Neuro-Kinesis’ (NKC) progress in forwarding the development of its Huygens™/Proteus™ Smart Catheter and Robotic Guidance System. The Huygens™/Proteus™ System is NKC’s lead product candidates for its line of SMART Surgical Tool technologies that combine advanced bio-data acquisition with enhanced Artificial Intelligence using proprietary micro-engineering and robotic-assisted control to provide surgeons an expanded tool-set to be able to deliver better surgical outcomes for their patients.
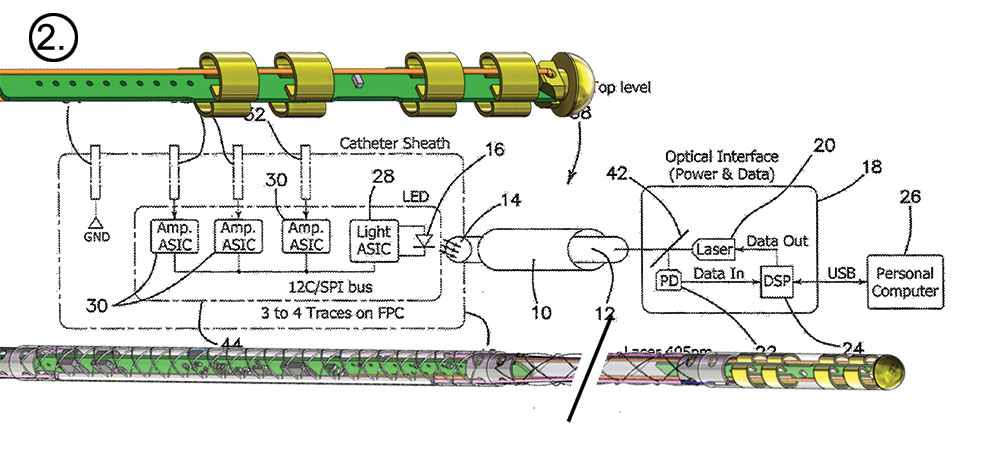
The Huygens™/Proteus™ System is specifically targeted to enhance the current state-of-art for the electro physiologist by providing a visionary catheter mapping technology that delivers a resolution capture that is 200x greater then current EP mapping catheters while also providing a robotic-assisted guidance systems for the catheter that produces a level of navigation control and return-to-point accuracy in a plug-and-play portable system that can help dramatically decrease procedural time and overall cost for the patient.
Wet Lab Testing Capabilities

NKC Engineers examine heart map generation on the EnSite NavX system
As previously announced, NKC has finalized its agreement with Sandia National Labs to conduct a clinical study looking at the signal acquisition capabilities of the Huygens™ Catheter’s locally-amplified electrophysiology catheter to capture and measure not only the higher voltage signals of traditional catheters, those that occur in the 100microvolt to 500microvolt (μV) range, but also those that occur in the low 5μV to 99μV where it is suspected that harder to detect small scar tissue cause complex arrhythmias. (Please see our Fourth Quarter Development Report for more details on the Sandia Study and the Disease model discussion below.)
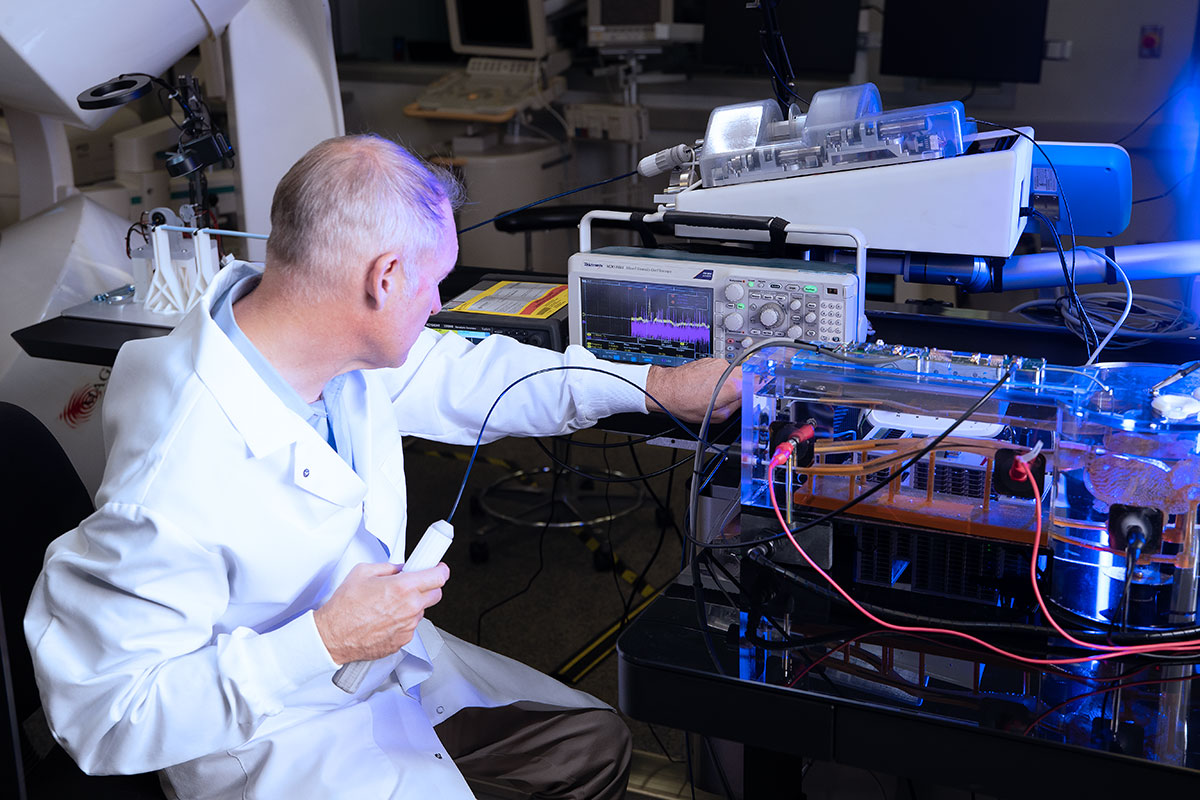
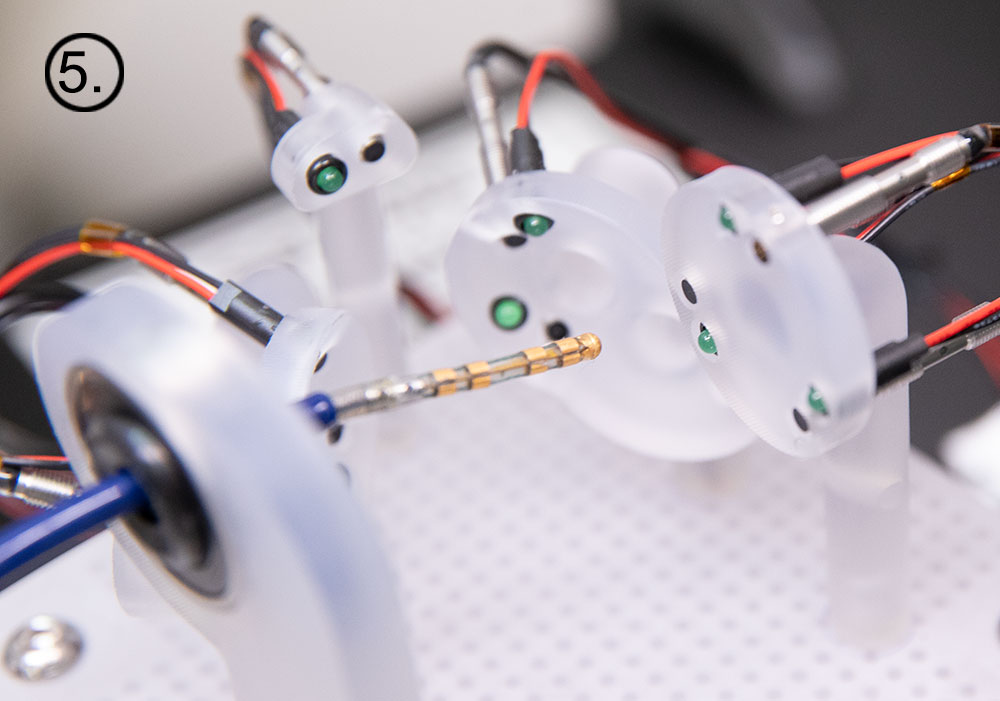
In preparation for the study scheduled to begin in Q123, NKC has installed a sophisticated Wet Lab testing platform to do preliminary data acquisition of several of the Huygens’ key functions. The first testing will be to ensure the ability of the catheter to interface successfully with the EnSite NavX system for trilateration position mapping.
Central to EP heart mapping is the ability of a catheter to deliver precision positioning data to create the dataset which is used in conjunction with traditional imaging such as fluoroscopy in order to determine an accurate 3D map of the geometry of a patient’s heart. To do this, five electrodes are placed on a patient’s body. Two are placed on the right and left side of the patient’s chest, one on the back of the neck, one on the lower back, and a final electrode on the patient’s inner thigh that acts as a ground. Small RF signals are then pulsed synchronistically from these electrodes. These signals
are picked up by the catheter inside the patient’s heart. By calculating the time difference between signal sent and signal received by the catheter sensor, the mapping software can triangulate a position in 3D space where the catheter is located. By repeating this process, and overlaying the results with the fluoroscopic image, the EP surgeon is able to build up a topographic map showing him the shape of the heart chamber walls as well as the location points other anatomical features such as valves, veins and arteries.
The Wet Lab provides the engineers a 1:1 scale model of a human heart along with a shortened section of the pulmonary vein so that catheter insertion, manual or robotic navigation, and trilateration positioning can be evaluated. At the time of this release, representatives from Abbott Medical have worked with the NKC engineers in setting up the initial interface systems between the standard catheters and the Huygens™ Catheters that are to be used in the Sandia study. The Abbott team has also provided the needed training for the engineers on the use of the NavX system while at the same time, the NKC engineers are giving the Abbott team a first look at the Huygens™ signal capture capabilities, the Proteus™ Robotic Arm’s navigation platform and the various other systems that comprise the Huygens™ / Proteus™ Operating Suite.


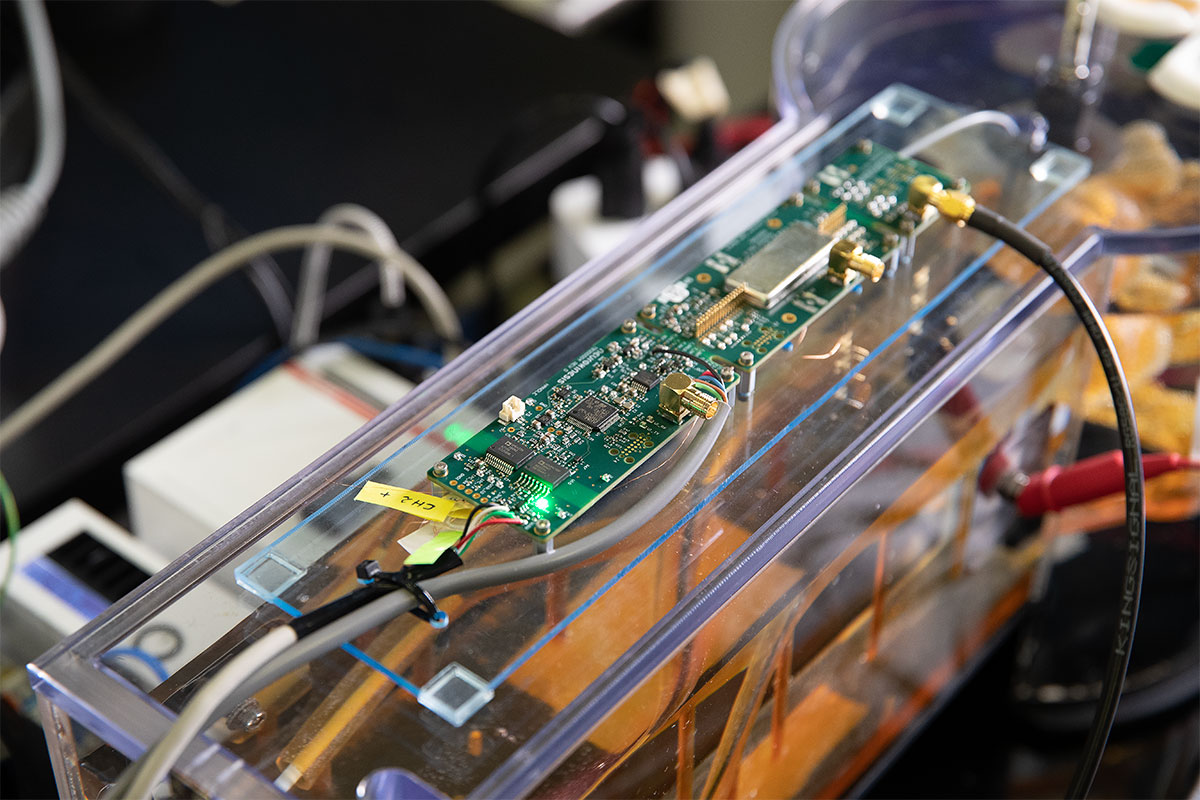

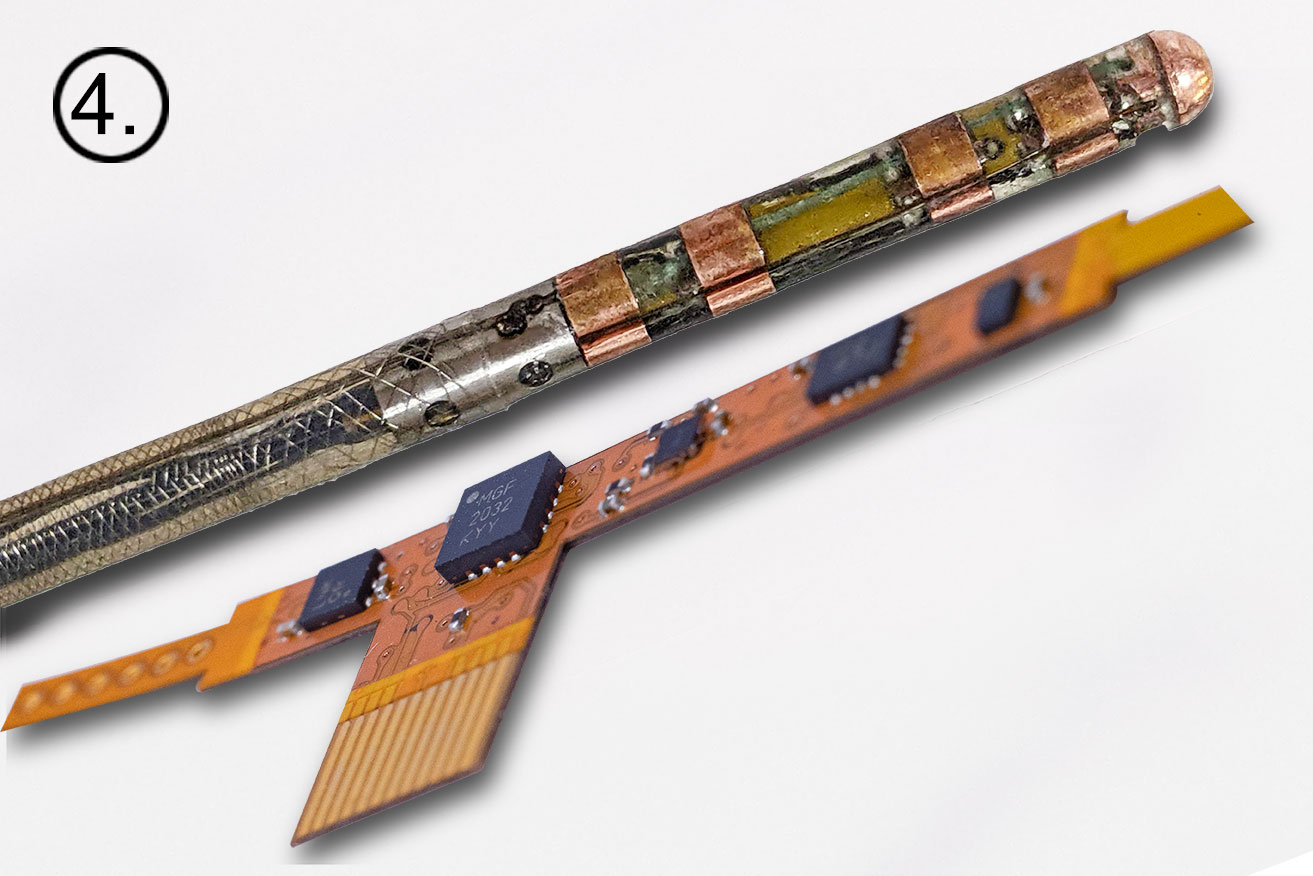
Images clockwise from top left: 1.) NKC Engineer inserting the Huygens™ Catheter into the Wet Lab as he sets up for testing the trilateration capabilities of the catheter. 2.) Image of the Huygens™ Catheter as it is being moved through the simulated pulmonary vein in the Wet Lab. 3.) The Huygens™ Catheter being maneuvered inside the heart model inside the Wet Lab. 4.) The test board that emulates the FCB inside the Huygens™ Catheter tip. This is the board that will be used in the testing to be done at Sandia National Labs.
The Strategic Regulatory Path Continues To Move Forward
As part of the regulatory strategy for NKC’s Huygens™ Catheter and Proteus™ Robotic Arm technologies, NKC is preparing to apply for its ISO-13485 Certification. The ISO-13485 certifies that a company has created and implemented a comprehensive quality management system for the design and manufacture of a medical device. Though not a requirement for manufacture, the ISO-13485 provides an awarded company market credibility and identity as to its adherence to both industry and governmental safety and best practice standards. The ISO-13485 is also used as the basis for obtaining foreign market certification as the designation is considered to be the in-line standard and requirement for medical devices. The European Committee for Standardization (CAN) uses the Quality Systems Management (QMS) standards defined in the ISO-13485 as a preferred method to prove compliance for medical device manufacturers seeking CE Certification. The ISO-13485 meets the Global Harmonization Task Force Guidelines (GHTF) guidelines which have become the universal standard for design, manufacture, export and sales of various medical devices which have also been also adopted by the newer International Medical Device Regulators Forum (IMDRF) protocols.
To achieve ISO-13485, a company must apply for the certification and then undergo a Stage 1 Pre-Certification Audit to evaluate the systems and procedures they have put in place in order to determine if there are any adjustments that need to be made. Once completed, and the company has made any required changes, a final Stage 2 Audit is performed after which the certificate can be granted.
To prepare for this audit, NKC has begun the development of its needed Standard Operating Procedure (SOP) documentation which both describes and provides the training and process description for every aspect of the manufacture and testing of each device platform. These documents are used for everything from parts acquisition and manufacture traceability, to assembly processes and device issue procedures. The SOPs are used both internally for the company’s testing and manufacture team as well as its strategic suppliers and Contract Manufacturing Organizations (CMO). Adoption of the SOPs ensure:
-
- the safety, health, environmental and operational processes and procedures necessary to perform a job properly are in place.
- that all production operations are performed consistently to maintain quality control of processes and
products. - scheduling and production timelines can continue uninterrupted and are completed on a prescribed schedule.
- that a streamlined process for handling process or production failures issues is in place to mitigate risk and
downtime concerns. - that comprehensive and compliant documentation is in place that shows adherence to all company and government regulations.
- training documentation about the process for which the SOP was written are consistent and minimize employee or strategic CMO error.
- that a Quality Assurance program checklist is in place for proper performance and manufacture.
- that a documented protocol is in place for auditors for financial, or regulatory compliance issues.
- a comprehensive historical record of the how, why and when each step of the manufactures, testing and engineering phase of a product is recorded.
NKC is anticipating it will be ready for it’s initial Stage 1 Pre-Certification Audit by the end of January 2023.
The upcoming testing at Sandia Labs will be to show that the Huygens™ Catheter accurately and consistently captures an array of electrical signals at all the levels of the dynamic range for a standard mapping catheter as well as the lower range ability specific to the Huygens™ Catheter. Testing of this capture is done by first calibrating the Huygens™ Catheter and a standard EP catheter to a U.S. Primary Standard of 1 microvolt which will guarantee that any reading subsequently measured by the catheters will have no clinically significant error variation between them. The images to the right show a variety of signals which are used to validate the Huygens™ Catheter’s performance. NKC is already performing in-house tests of the Huygens™ in both a bench-top and Wet Lab environment to ensure the technology will be able to successfully pass the Sandia validation study.
The images below show some of the daily activity underway at NKC as development of the Huygens™/Proteus™ technology accelerates in order to meet the milestone paths set for 2023. From left to right: 1.) An engineer at the wet-lab manually navigates the Huygens™ Catheter into position to perform trilateration testing. 2.) Calibration of the signal generator for trilateration testing is calibrated. 3.) Members of the engineering team test the various Operating Suite control systems. 4..) Josh Shachar joins the engineering team in the Animal Lab as they perform some of the EnSite NavX integration work.



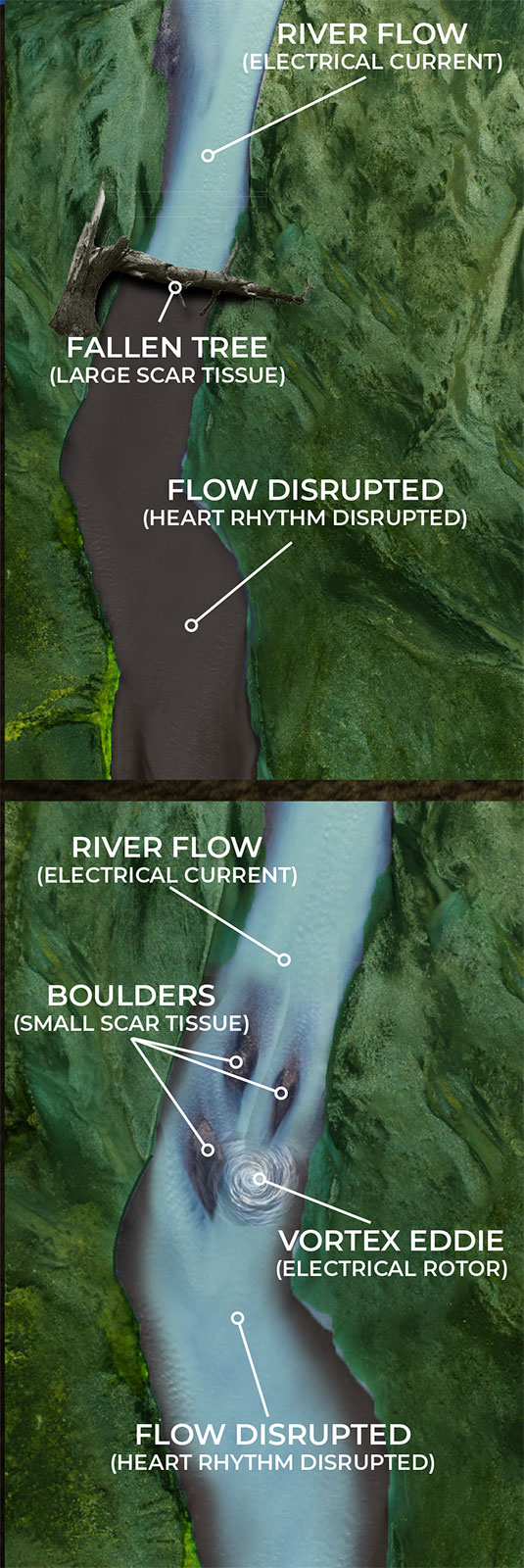
THE RIVER ANALOGY
When trying to describe EP-based arrhythmias, no analogy is perfect, but the following provides some base understanding of what NKC is working to achieve.
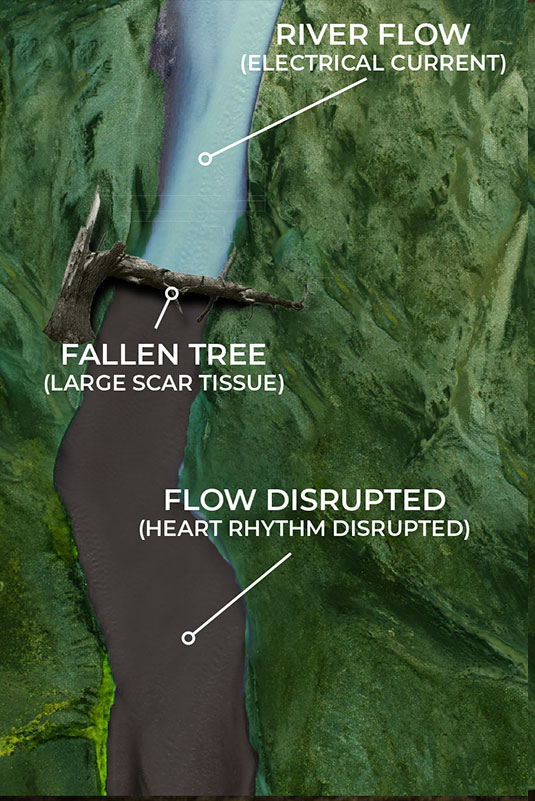
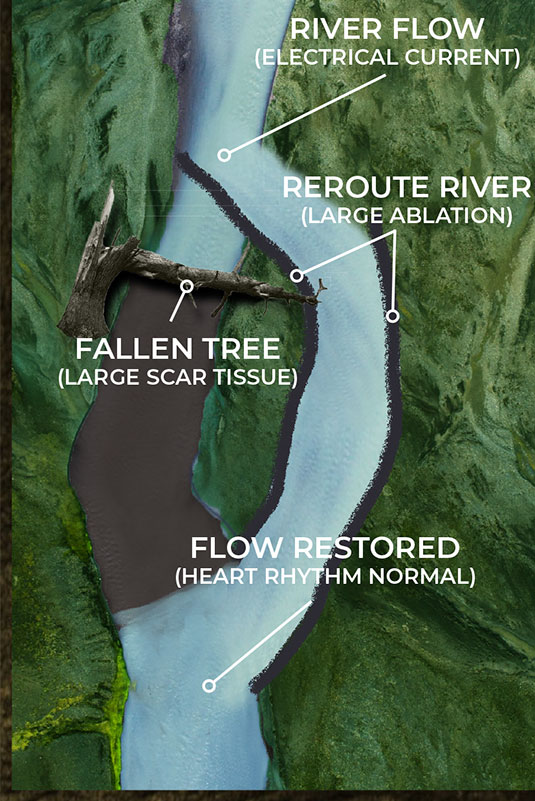
Current EP mapping is able to see the large voltage electrical activities that are creating disturbances in the pacing of the heart. This is shown by the illustrations on the top left and right where a tree has fallen across a river completely blocking the flow of water. The tree in this analogy is the equivalent of a large scar tissue that is readable in the ≥ 100µV range. Problems like this are easy to see and easy to fix as the surgeon simply can make very large ablation scars that in essence create a new channel for the water to be redirected around the disturbance and back into the river bed downstream.
But what happens when the scar tissue is small and unreadable by the current mapping technology? The EP surgeon knows there is a problem, they are just not able to see it. The NKC Huygens™ Catheter is able to see these scars as it can read in the ≤100µV range.
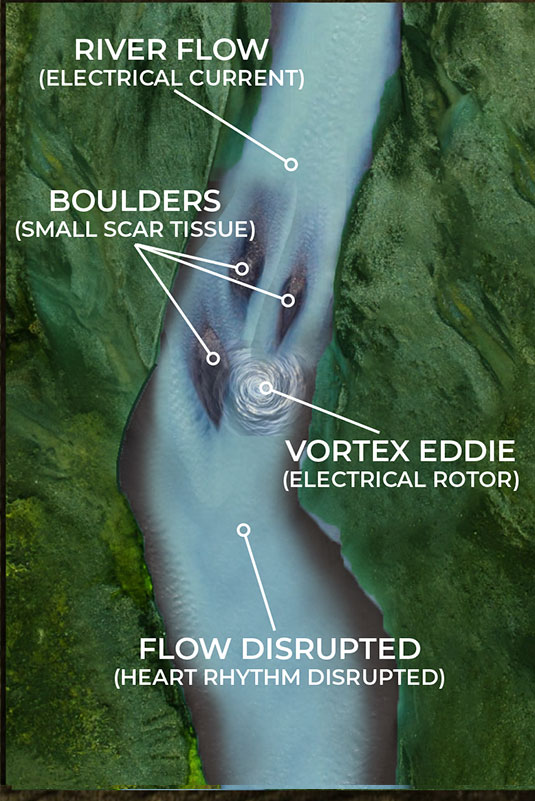
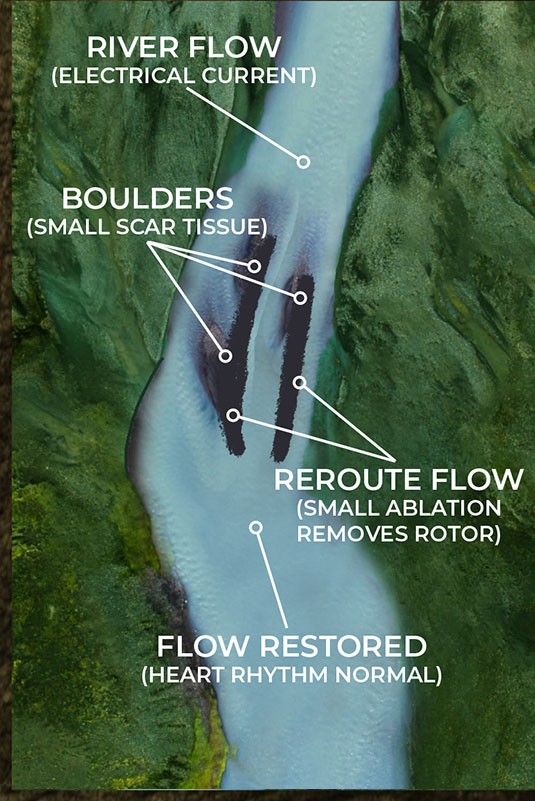
As the illustrations on the lower left and right show, small scar tissue is not blocking the current completely, but it is causing the electrical energy to form eddies in the flow; an energy vortex. These vortexes are knows as rotors in the EP world. This disruption is not enough to stop the flow of current but it is sufficient to diminish and disrupt it enough to cause arrhythmia issues.
By being able to identify these rotors in complex arrhythmia cases, the Huygens™ Catheter will allow the EP Surgeon to approach ablation much more surgically by just creating small ablation scars that will eliminate the small scar tissue from creating these eddies of electrical energy.
If successful, this ability and the new approach to ablation that can be provided, will fundamentally change the face of diagnostic electrophysiology.
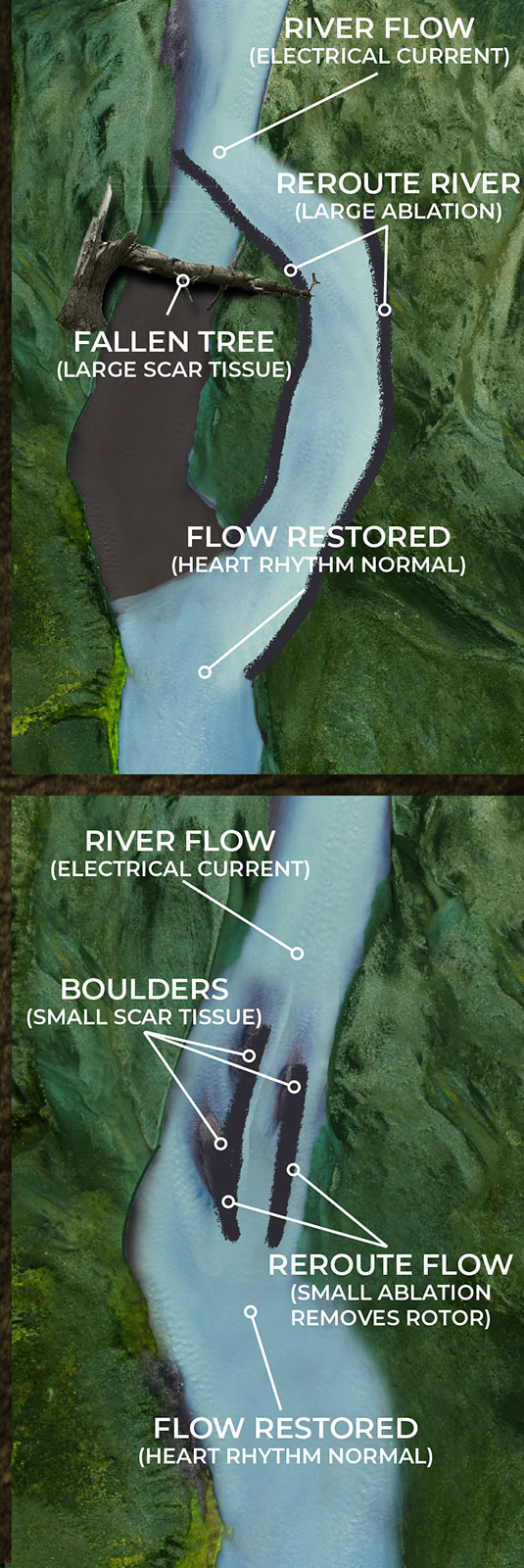
THE RIVER ANALOGY
When trying to describe EP-based arrhythmias, no analogy is perfect, but the following provides some base understanding of what NKC is working to achieve.
Current EP mapping is able to see the large voltage electrical activities that are creating disturbances in the pacing of the heart. This is shown by the illustrations below where a tree has fallen across a river completely blocking the flow of water. The tree in this analogy is the equivalent of a large scar tissue that is readable in the ≥ 100µV range. Problems like this are easy to see and easy to fix as the surgeon simply can make very large ablation scars that in essence create a new channel for the water to be redirected around the disturbance and back into the river bed downstream.
But what happens when the scar tissue is small and unreadable by the current mapping technology? The EP surgeon knows there is a problem, they are just not able to see it. The NKC Huygens™ Catheter is able to see these scars as it can read in the ≤100µV range.
As the illustrations below show, small scar tissue is not blocking the current completely, but it is causing the electrical energy to form eddies in the flow; an energy vortex. These vortexes are knows as rotors in the EP world. This disruption is not enough to stop the flow of current but it is sufficient to diminish and disrupt it enough to cause arrhythmia issues.
By being able to identify these rotors in complex arrhythmia cases, the Huygens™ Catheter will allow the EP Surgeon to approach ablation much more surgically by just creating small ablation scars that will eliminate the small scar tissue from creating these eddies of electrical energy.
If successful, this ability and the new approach to ablation that can be provided, will fundamentally change the face of diagnostic electrophysiology.
Defining The Disease
In the previous press release, NKC announced its regulatory strategy to seek Humanitarian Use Device (HUD) classification for its Huygens™ Catheter technology. To review, the HUD classification would provide a much shorter path to human clinical trial use. To qualify, NKC needs to show two things. The first is that there exists a small population of patients who have a need that is not being met via any existing process or device; and that the development of such a process or device for treating these patients is impossible, highly impracticable, or unsafe. The second is that the manufacturer’s process or device can provide at minimum, a rate of improvement or cure that meets or exceeds current treatment standards, and that their device or process is safe to use and does not create any unknown secondary issues.

Dr. Eli Gang, MD, FACC, FACP NKC Chief Medical Officer
As mentioned in the prior release, Chief Medical Officer Dr. Eli Gang is working along with the NKC Regulatory team to gather the initial data sets to show that there is an under-served population of patient’s with complex arrhythmias originating within scars in the lower chambers of the heart (ventricles). Many if these ventricular arrhythmias originate from, or are driven by, scars in the ventricles formed by prior infarctions or other disease entities causing damage to the heart muscle. These arrhythmias (ventricular tachycardia) are perpetuated by currents which enter and exit the scars in a repetitive manner. It is the task of the physician to map the scar and to find, when possible, the entrance or exit points of the ‘channels’ within these scars. The challenge confronting physicians attempting to treat these arrhythmias is the known observation that ventricular scars generate very small electrical signals (frequently less than 50µV). The signals of interest also include prolonged, high frequency ‘diastolic potentials’ which are thought to show slow conduction within scars and are considered to be excellent ablation target sites for curative ablations, when confirmed to be within the all-important channels responsible for perpetuating the rhythm. At times, using current available technology, these crucial signals of interest are barely larger than the background noise generated by the recording systems. The Huygens™ Catheter will overcome these recording challenges by enabling the physician to see very small signals with much improved S/N ratio, i.e., vastly enhanced signal clarity free of background noise generated by ambient electromagnetic noise (EMI).
In other chambers of the heart such as the atria, other abnormal heart rhythms not directly related to large scars frequently occur. Normal, rapidly conducting tissue will generate large electric signals (> 100-200µV). Here, too, large circuits can be delineated with careful mapping (in rhythms such as atrial flutter) and the trick is to find the areas which would best terminate these large repetitive circuits. Such target areas frequently manifest small and repetitive signals, suggesting slow conduction within anatomic boundaries, analogous to a stream flowing between two large boulders. Interruption of the flow terminates the abnormal rhythm. Yet at other times, multiple vortices are created by inhomogeneous flow in fibrillating atria, analogous to the stones within the stream causing the so-called ‘rotors’ which are thought to perpetuate atrial fibrillation. The Huygens™ Catheter is designed to correctly delineate these important catheter ablation target sites, thus enabling the physician to effectively terminate abnormal cardiac arrhythmias. This should improve the efficacy and safety of these procedures by enhancing the accuracy of the ablation, minimizing the mapping time, shortening overall procedure duration, and increasing patient safety.
Recent discussion with CEO and technology inventor Josh Shachar sheds lights on the problem of small scar tissue electrical disruption in the creation of rotor events which are thought to be a factor in complex arrhythmia cases.

Josh Shachar tests operation of the Proteus™ Robotic Arm with the Huygens™ Catheter.
“When arrhythmia is caused by a large disruption of the ability of the electrical signal to pass efficiently through the endocardial tissue, our current large ablation approach can be very effective.” stated Josh, “Though not a perfect analogy, you can think of it as a massive boulder falling across a river, stopping the flow of water. A large ablation in essence is creating a new channel or pathway for the whole river to detour around the stone to start flowing again.
“Over the past several years though,” continued Josh, “more and more studies are showing that many arrhythmic events could by caused by what are called rotors. Rotors occur when the propagation of an electrical wavefront, like a heart pacing signal, encounters in-excitable tissue causing the signal to distort and begin to rotate around the tissue in a vortex-like fashion. These rotors and focal impulses have been proposed to be the underlying drivers of a significant portion of atrial fibrillation (AF) in humans.
“Coming back to our river analogy, instead of a large stone, like large scar tissue, blocking the flow, the river’s current is being disrupted by a series of small boulders. These boulders do not stop the flow completely, but they disrupt the energy of the flow by causing the water to begin to eddy just underneath the boulder, thus creating a disturbance and decrease in the current.
With the Huygens™ Catheter, we intend to show that we can give the EP Surgeon the ability to see these rotors, or small microvolt disruptions. In doing so, the EP Surgeon can change the ablation strategy to just focus on rerouting the current around the boulder, instead of having to divert the whole river.
The efficacy this would bring in better outcomes for patients with complex arrhythmia issues, the decrease in time and cost in locating issues and ablating them, would be tremendous. Demonstrating this with our Huygens™ technology could be a game changer in Electrophysiology.”
The journey from dream to reality for the Huygens™ Catheter represents over a decade of effort by the NKC team. The images below give a brief look from initial concept to a real world advanced mapping catheter that has the potential to advance catheter-mapping into the future. 1.) Huygens’™ origins lie in the development of the MOSFET Catheter in 2012. 2.) Early schematics of the Huygens™ Catheter show the significant shift in design. 3.) This is an early 3D concept rendering of the Huygens™ Catheter in 2918. 4.) A close-up of the current design of the Huygens™ Catheter and the embedded FCB board. 5.) The Huygens™ Catheter undergoing initial targeting tests in early 2022. 6.) The Huygens™ Catheter being inserted in the NKC Wet Lab testing model. 7.) The Huygens™ Catheter inside the simulated heart model for trilateration testing. 8.) Josh Shachar holding the Huygens™ Catheter mounted into the Proteus™ Catheter Handle inside the operational NKC Animal Lab that contains the NKC Operating Suite.





SAFE HARBOR DECLARATION
THE STATEMENTS, PROJECTIONS AND ESTIMATES OF FUTURE PERFORMANCE OF THE COMPANY OR VARIOUS ELEMENTS OF THE COMPANY’S BUSINESS CONTAINED IN THIS MEMORANDUM THAT ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD EXPECT THAT ANTICIPATED EVENTS AND CIRCUMSTANCES MAY NOT OCCUR, THAT UNANTICIPATED EVENTS AND CIRCUMSTANCES WILL OCCUR, AND THAT ACTUAL RESULTS WILL LIKELY VARY FROM THE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD BE AWARE THAT A NUMBER OF FACTORS COULD CAUSE THE FORWARD-LOOKING STATEMENTS OR PROJECTIONS CONTAINED IN THIS MEMORANDUM OR OTHERWISE MADE BY OR ON BEHALF OF THE COMPANY TO BE INCORRECT OR TO DIFFER MATERIALLY FROM ACTUAL RESULTS. SUCH FACTORS MAY INCLUDE, WITHOUT LIMITATION, (i) THE ABILITY OF THE COMPANY TO COMPLETE THE DEVELOPMENT OF ITS PRODUCTS IN A TIMELY MANNER, (ii) THE DEMAND FOR AND TIMING OF DEMAND FOR SUCH PRODUCTS, (iii) COMPETITION FROM OTHER PRODUCTS AND COMPANIES, (iv) THE RESULTS OF THE COMPANY’S SAFETY AND EFFICACY STUDIES, (v) THE RESULTS OF THE REGULATORY APPROVAL PROCESS, (vi) THE COMPANY’S SALES AND MARKETING CAPABILITIES, (vii) THE COMPANY’S ABILITY TO SELL ITS PRODUCTS PROFITABLY, (viii) THE ABILITY OF THE COMPANY’S THIRD-PARTY SUPPLIERS TO PROVIDE PRODUCTS AND SERVICES IN A RELIABLE MANNER; (ix) AVAILABILITY OF ADEQUATE DEBT AND EQUITY FINANCING, AND (x) GENERAL BUSINESS AND ECONOMIC CONDITIONS. THESE IMPORTANT FACTORS AND CERTAIN OTHER FACTORS THAT MIGHT AFFECT THE COMPANY’S FINANCIAL AND BUSINESS RESULTS ARE DISCUSSED IN THIS MEMORANDUM UNDER “RISK FACTORS.” THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL BE ABLE TO ANTICIPATE, RESPOND TO OR ADAPT TO CHANGES IN ANY FACTORS AFFECTING THE COMPANY’S BUSINESS AND FINANCIAL RESULTS.

