FOR IMMEDIATE RELEASE
NKC Releases 2023 Third Quarter Development Report on the Huygens™/ Proteus™ Technology
DATELINE: November 07, 2023, Los Angeles, CA
Neuro-KInesis, Corp. (NKC), is providing the following to share the 2023 third quarter updates on Neuro-Kinesis’ (NKC) progress in forwarding the development of its Huygens™/Proteus™ Smart Catheter and Robotic Guidance System. The Huygens™ Catheter and the Proteus™ Robotic Arm are NKC’s lead product candidates for its line of SMART Surgical Tool technologies that combine advanced bio-data acquisition with enhanced Artificial Intelligence using proprietary micro-engineering and robotic-assisted control to provide surgeons an expanded tool-set to be able to deliver better surgical outcomes for their patients.
WET LAB UPDATE

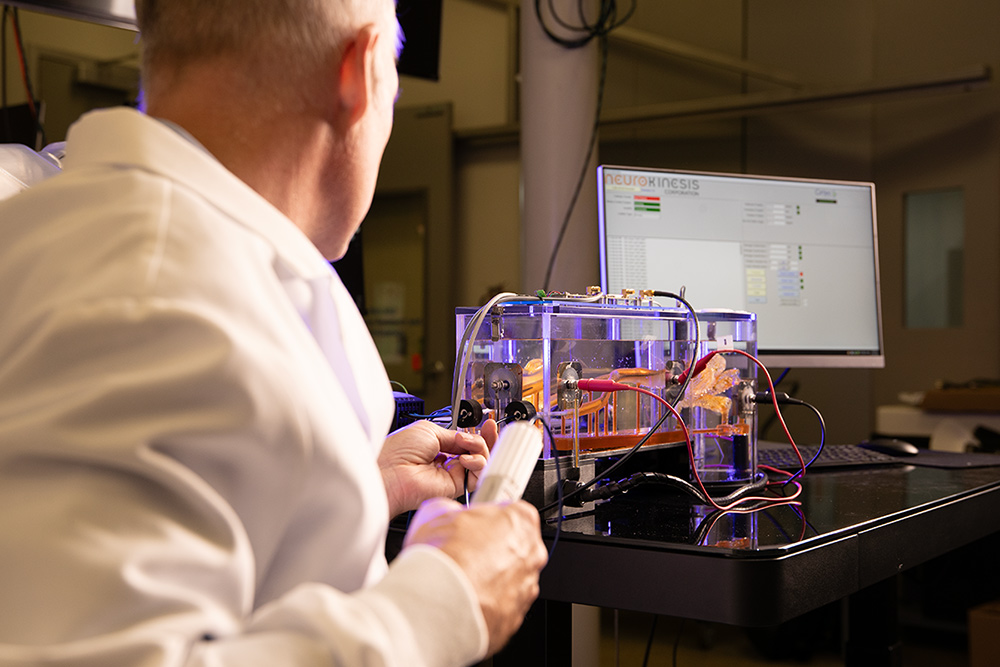
NKC Engineer connecting trilateration leads to the Wet Lab
In the previous progress report, NKC announced the installation of its wet lab capabilities at its Los Angeles Headquarters. The wet lab provides the company the ability to be able to do in-house testing of the Huygens™ Catheter in a 1:1 scale replica of a human heart and a shortened section of the pulmonary vein. The wet lab allows the engineering team to be able to do catheter insertion, manual or robotic navigation, and trilateration position testing of the catheter.
In an actual EP heart-mapping procedure, the position of the catheter tip is tracked using a technique known as trilateration. In the process, the EP physician places five electrodes on the patient’s body in the following locations: (2) on the right and left side of the patient’s chest, (1) on the back of the neck, (1) on the lower back, and (1) on the patient’s inner thigh that acts as a ground. Small RF signals are then pulsed synchronistically from these electrodes. These signals are picked up by the catheter inside the patient’s heart. By calculating the time difference between the signal sent and the signal received by the catheter sensor, the mapping software can triangulate a position in 3D space where the catheter is located. By repeating this process, and overlaying the results with the fluoroscopic image, the EP surgeon can build a surface map of the potential reading at each location in the heart, showing the shape of the heart chamber walls as well as the location points of other anatomical features such as valves, veins, and arteries.
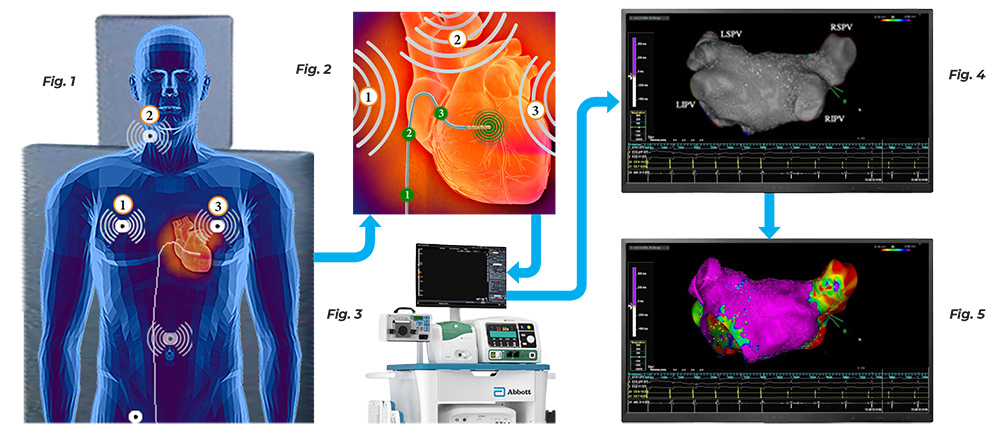
In Electrophysiology, the process of trilateration detection is fundamental in obtaining an accurate map of a patient’s heart. Figure 1 shows how electrodes are place at precise locations on a patient chest, back, neck and groin. Once the EP mapping catheter is in position in the heart, these electrodes emit consecutive RF impulses which are picked up by the catheter tip. Figure 2 shows the catheter tip acquiring each signal as it arrive and sending that detection back to the heart mapping computer. Figure 3 shows the signal arriving at the heart mapping computer, in this case the Ensite Nav X, where the timing between when the electrode sent a signal and when the catheter picked up the signal is computed and analyzed. Figure 4 shows how these data points as they are collected begin to build up the X,Y, Z coordinates of where the catheter was at the point of detection and this allows the computer to develop the topographic map of the heart. Because there is no bio-impedance or bioconductivity measurements being done, this on it s own will only yield a gray scale surface map. Figure 5 shows how when trilateration location detection is combined with bio-impedance and bioconductivity data capture, then the EP physician can now see the surface but also the quality of the tissue and its ability to conduct the electrical energy needed for proper heart pacing.
To be able to do its simulation testing, as well as its ongoing research and development of the Huygens™ Catheter’s signal acquisition capabilities, NKC has installed the Abbott/St. Jude EnSite NavX Heart Mapping system, which is the current gold standard for 3D heart mapping. Since its installation, the NKC team has worked with representatives from Abbott/St. Jude in establishing the interface between the EnSite NavX heart mapping system and the Huygens™ Catheter to establish trilateration testing protocols to confirm the ability of the catheter to capture the positioning data for creating a detailed 3D map of the geometry of the heart’s interior walls and chambers.
The NKC wet lab simulates this by positioning five selectively placed electrodes positioned on the outside of the wet lab’s tank through which the timed RF signals are pulsed from the EnSite NavX system. With the help of the Abbott / St. Jude team, the NKC engineers have been able to perform all the baseline testing of the system using a standard spiral mapping catheter to confirm the system has been calibrated to the necessary standards. As a result, the team has been able to successfully generate several trilateration mapping simulations using the standard catheters and the NavX. Testing of the system with the Huygens™ Catheter is anticipated to begin once the next generation prototype of the Huygens™ is delivered in November.


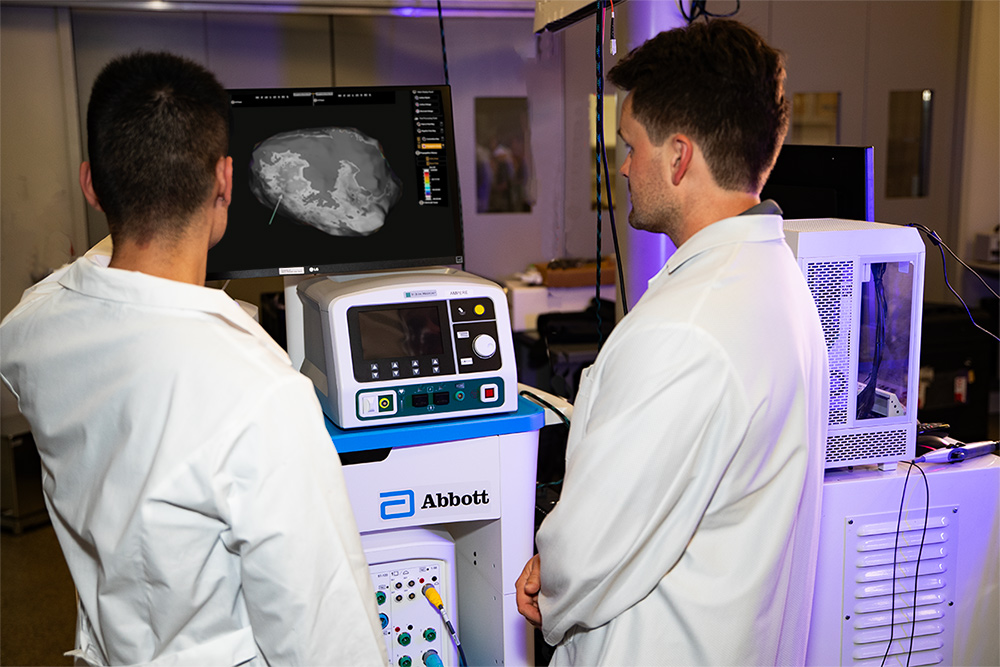
The images above show the wet lab /EnSite interface testing where an Orion catheter was used to generate the topographical map of the simulated heart.
SANDIA TESTING
As previously announced, NKC had formalized an agreement with Sandia National Labs to conduct a series of tests of the Huygens™ Catheter as it approaches the final prototype manufacturer for the upcoming animal study later this year. The initial test protocols outlined were to establish the Huygens™ Catheter’s ability to accurately capture and transmit specific generated signals to a computer display that would demonstrate an ability equal to that of existing state-of-the-art catheters.
To do this, the NKC engineering team created a bench-top test board that replicates the electrode capture array and signal processing routine that is embedded in the Huygens™ flexible-circuit board (FCB) in the catheter’s tip. The test board allows Sandia technicians to connect a signal generator to the electrode points and then send calibrated and standardized test signals to the board. These signals are then processed as they would be in an actual EP heart mapping procedure by amplifying and filtering the signal to remove noise and artifacts and then converting the analog signal to a digital format which is then sent to a computer display to be analyzed in comparison to the generated signals fed to a calibrated oscilloscope.
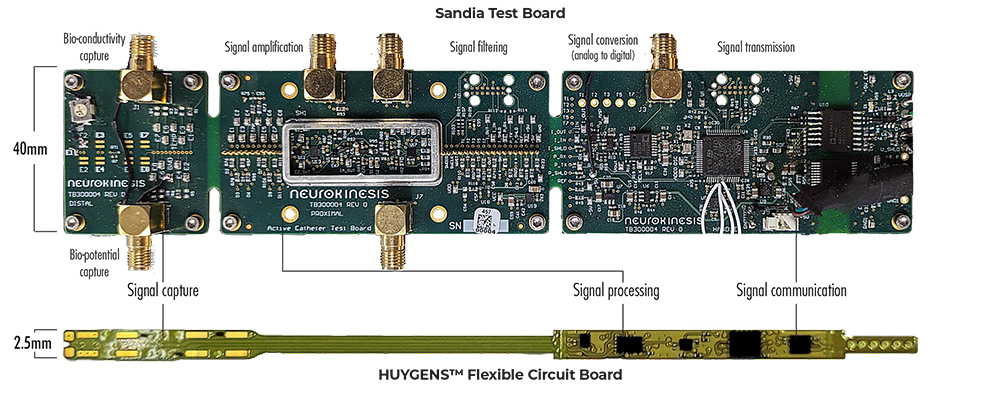
The Sandia Test Board was created to provide a bench-top replica of the Huygens™ Catheter’s flexible circuit board (FCB) for validation testing of the technology for both continued research and development and for submission to the FDA for regulatory approval. The Sandia Test Board allows controlled test signals to be captured, processed, and outputted to both an analog reader, such as a calibrated oscilloscope, or to a digital display.
HUYGENS™ CATHETER DEVELOPMENT
With the first pre-clinical animal study of the Huygens™ Catheter scheduled to begin in Q1-2024 at the Technion Institute in Israel, the NKC engineering team is rapidly working to finalize the final design requirements for the Huygens™ Catheter FCB, the catheter handle and the communication interface to have NKC’s catheter fabrication partner Seisa Medical time to produce the 20 prototypes needed for the study.
For the study, the team will be looking to validate the ability of the catheter to capture live animal biopotential cardiac signals as well as being able to confirm that the catheter meets all the FDA requirements for safety as a Class II device. Most all the milestones required for the prototype version of the Huygens™ Catheter for the animal study have been completed and the team is focused on validating the ability of the catheter to provide accurate bioimpedance measurements in the environments of the defined test protocols.
Traditional EP heart mapping involves gathering two types of data. The first is to measure where electrical current is flowing and where it is not. This is done by measuring the biopotential of the heart tissue, In essence, this is looking at the surface of the heart tissue to see where the current is flowing, Biopotential readings can tell you “where” the current is moving but it cannot tell you “why” the current is being blocked or slowed down. To do that, the EP physician must also collect bioconductivity reading which looks at the quality of the tissue below the surface to see what may be causing disruption.
Bio-potential Readings
The image below shows a topographical look at myocardial tissue in a patient’s heart. As can be seen, where there is healthy tissue, electrical signal and current for normative heart pacing can flow cleanly through the tissue that lies between the two red markers. However, where the is damaged and/or scarred tissue, electrical current cannot flow smoothly, if at all once scar tissue becomes deeply embedded.

Healthy Tissue
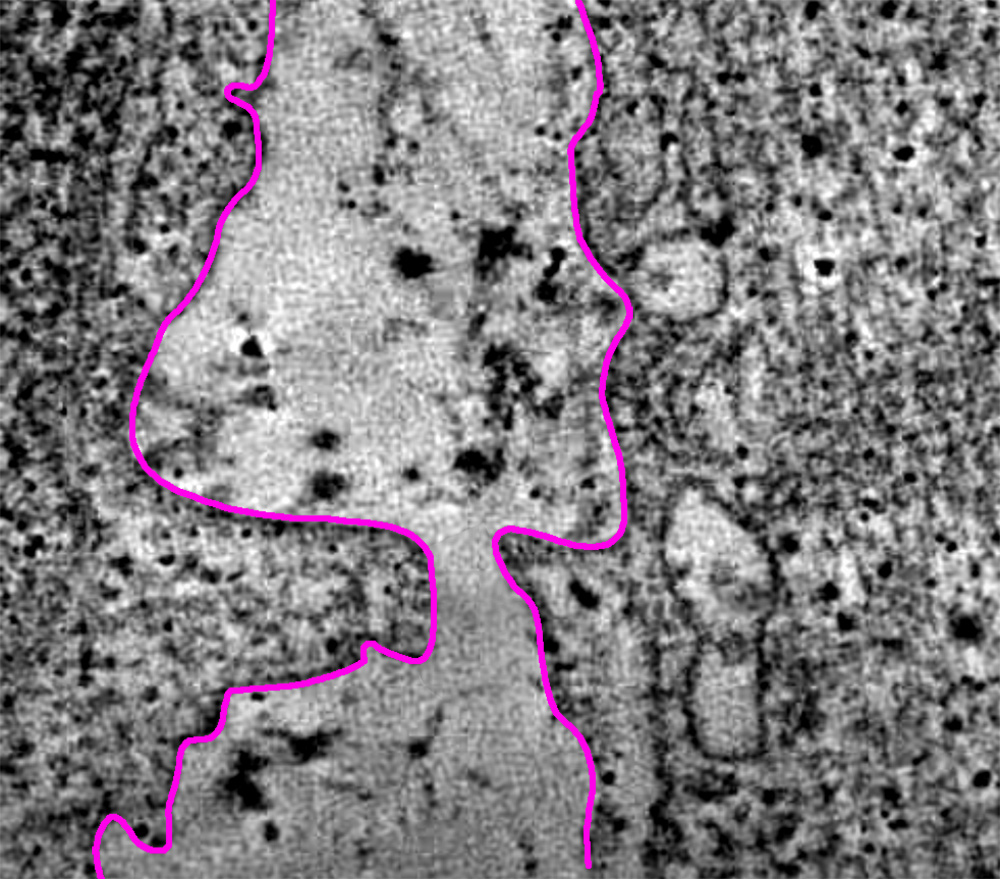
Surface view of heart tissue showing a channel of healthy tissue that is flanked by heavily scarred tissue.

Scar Tissue
Bio-conductivity Readings
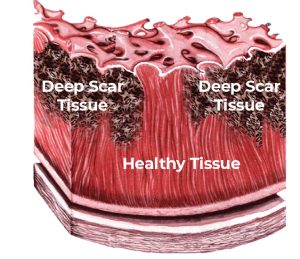 To look below the surface to see what the quality of the tissue is, the EP physician must measure the bioimpedance of the tissue. This is done by sending a small electrical voltage into the tissue and measuring the resistance or impedance of the tissue to the electrical pulse, As one can imagine, the thicker and deeper the scar tissue, the harder it is for any electrical current to travel through that space. By doing both bio-potential measurements along with bio-conductivity measurements, the EP physician can build a much clearer map of the patient’s heart to determine the proper therapeutic course for corrective action, such as an ablation, to restore proper sinus pacing.
To look below the surface to see what the quality of the tissue is, the EP physician must measure the bioimpedance of the tissue. This is done by sending a small electrical voltage into the tissue and measuring the resistance or impedance of the tissue to the electrical pulse, As one can imagine, the thicker and deeper the scar tissue, the harder it is for any electrical current to travel through that space. By doing both bio-potential measurements along with bio-conductivity measurements, the EP physician can build a much clearer map of the patient’s heart to determine the proper therapeutic course for corrective action, such as an ablation, to restore proper sinus pacing.
Huygens™ Catheter Vision
Since its inception, NKC’s goal has been to push the potential of existing medical device technology into new realms of both diagnostic and therapeutic capabilities. As such, the engineering team has had to define new solutions for technical issues that have never been encountered to achieve the innovation goals established for each platform the company creates. And at each such challenge, the team has been successful in finding answers that move the boundaries of possibility forward.
The Huygens™ Catheter technology was created to expand the potential of EP mapping in three main areas. The first was to be able to dramatically reduce the issues of signal-to-noise ratio contamination that currently limits the art. When measuring high-voltage biopotential signals, noise is not the major limiter; however, for signals that occur below 500 microvolts, degradation of the signal due to environmental noise sources becomes a major concern. The Huygens™ Catheter solves this issue by using FCBs located within the lumen of the catheter to digitally process the biopotential signal close to the location of measurement.
The third goal envisioned in the technology was to shorten the time of electrophysiology procedures. To help with this, the NKC team wanted to provide the EP physician with the ability to perform both biopotential and bioconductivity mapping at the same time; a process that currently is separated into two procedures where bioconductivity mapping is often eliminated because of the inherent time factors involved. As such, this important data is often not available when the physician is making a therapeutic decision, such as where and how to ablate.
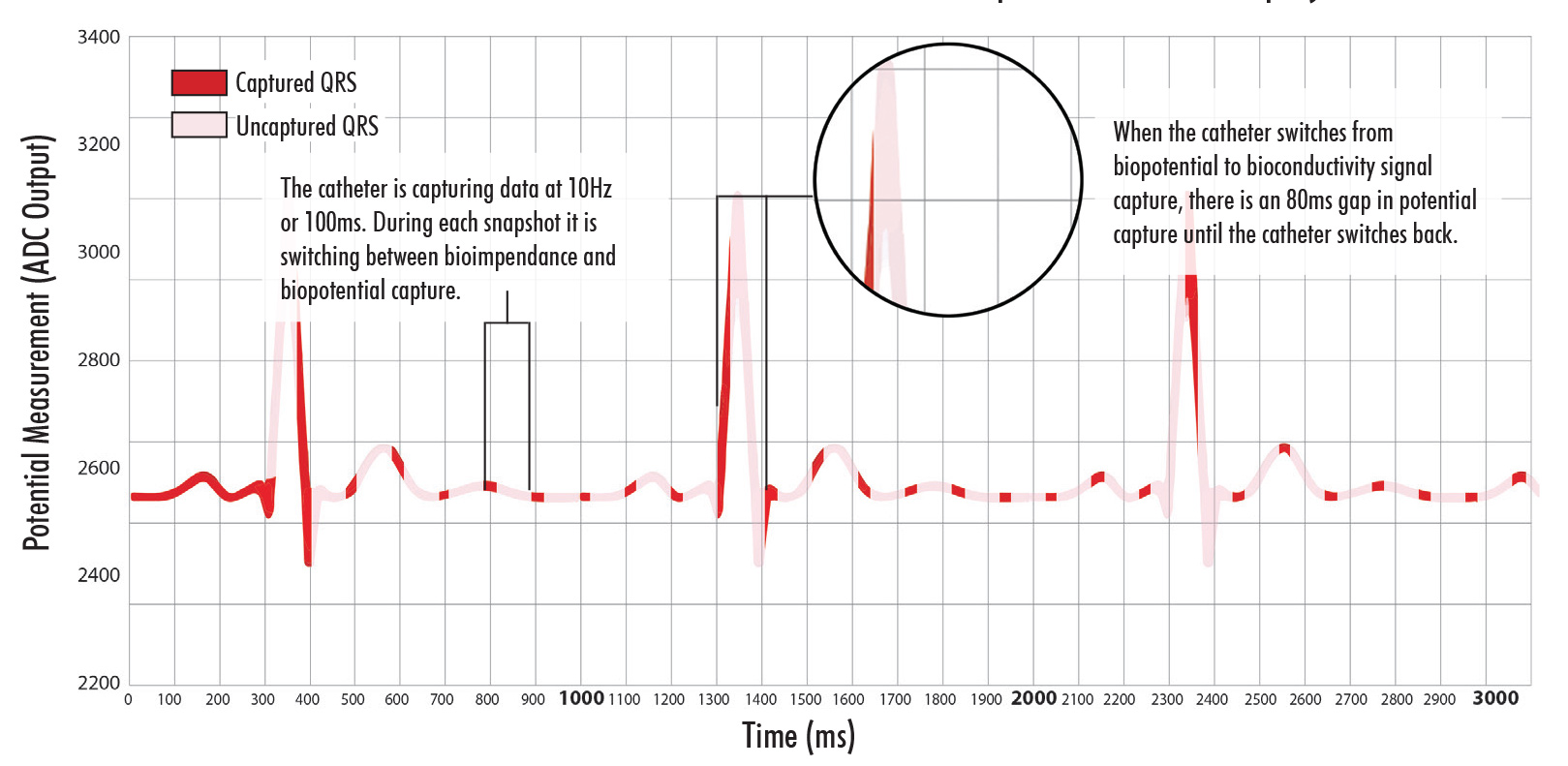
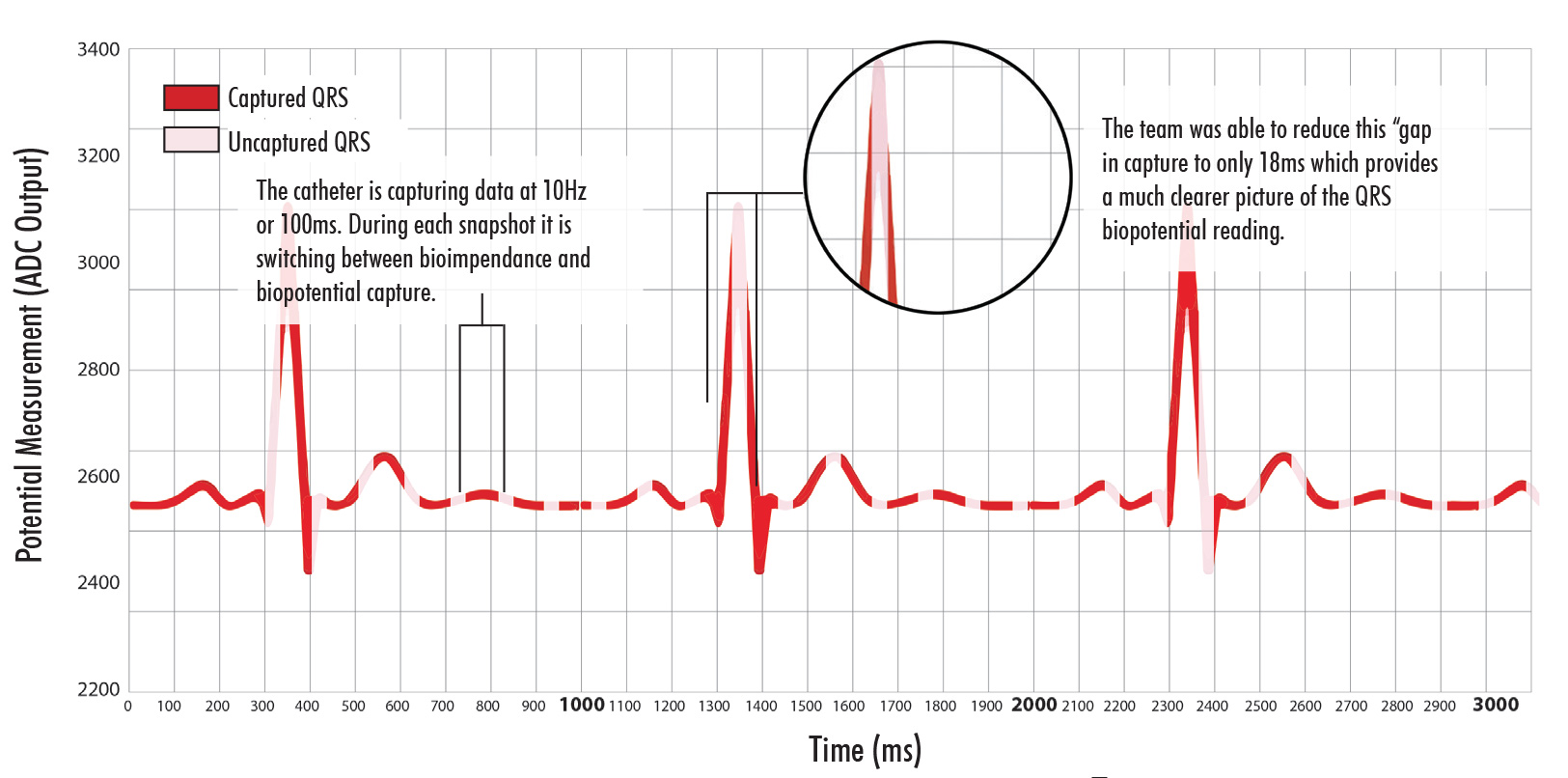
These graphs show the solution the NKC team made in closing the signal capture gap when switching between biopotential testing and bioconductivity testing. The top chart shows the 80ms gap that was seen when the catheter switched between measuring modes. As can be seen in the lower graph, the team has been able to reduce that gap down to 18ms which eliminates concern for loss of statistically relevant biopotential data capture occurring during this period. Further, the team has eliminated the gap issue completely by providing the EP physician manual control over data capture modes.
Delivering Biopotential and Bioconductivity Mapping in One Procedure
In biopotential mapping, the catheter measures the electrical potential of the myocardial tissue by making direct contact with the surface of the tissue. This in essence provides the EP physician a look at the topography of the heart as well as where the electrical current is moving on the surface of the tissue. To analyze the subsurface structure of scars in the myocardial tissue, the Huygens™ Catheter performs bioconductivity measurements during mapping in addition to traditional biopotential measurements. In this process, the electronics on the FCB switch between measuring bioconductivity and biopotential once every 100 ms. In biopotential mode, the voltage at the myocardial surface is measured at each electrode as in a traditional EP catheter; in biopotential mode, the catheter injects electrical current into the tissue to measure the resistance thereby determining the conductivity of the bulk tissue.
The Huygens™ Catheter will be the first catheter on the market to combine both of these measurements into a single tool.
Closing the Gap

The image here shows the PM Phillips data communication assembly that will interface the Proteus™ Robotic Handle handle with the Ensite NavX.
In the Sandia testing it was discovered that there was a short gap of 80 milliseconds as the electronics in the FCB switched between the two modes. During this time, the catheter is not able to capture a signal. In heart mapping even this short of a time gap in measurement could cause critical anomalies in the electrocardiogram to be missed.

The above image shows the KUKA Robot in place at the NKC Animal Testing Lab in Los Angeles, CA.
This problem has never been addressed up until now because traditional EP catheters do not measure biopotential and conductivity in this way. But as they have done countless times before, the NKC team set about finding a solution to the issue in order to achieve its goal. The team addressed this issue in two ways: 1) by reducing the conductivity mapping time from 80 to 10 milliseconds (a 70% decrease) to minimize the amount of potential data loss, and 2) by providing the user the ability to switch off the impedance measurement, so that there are no gaps in the potential measurement between bioimpedance only, biopotential only, and auto-switching data capture.
The team, led by CEO and CTO Josh Shachar is now working with CMO Dr. Eli Gang to determine if this improvement now meets the standard to be able to deliver clinically relevant data for both anatomical and substrate mapping. If it does, the team will have once again demonstrated its ability to turn vision into reality.
Huygens Catheter Shaft
In addition to this work, the engineering team has made advancements in several further development milestones. This includes refinement and finalization of the design for the Huygens™ Catheter shaft. The shaft is a critical component in the catheter as it provides the conduit by which all data communication is done, all power is supplied, and through which the mechanical adjustments to catheter tip translation are made. The catheter shaft is a 45” long conduit made of biocompatible medical-grade plastic which encases the catheter’s guide wires and power/communication cabling. On the proximal end, the sheath is joined to the catheter tip where the connections are made to the FCB, and on the distal end to the catheter handle control board.
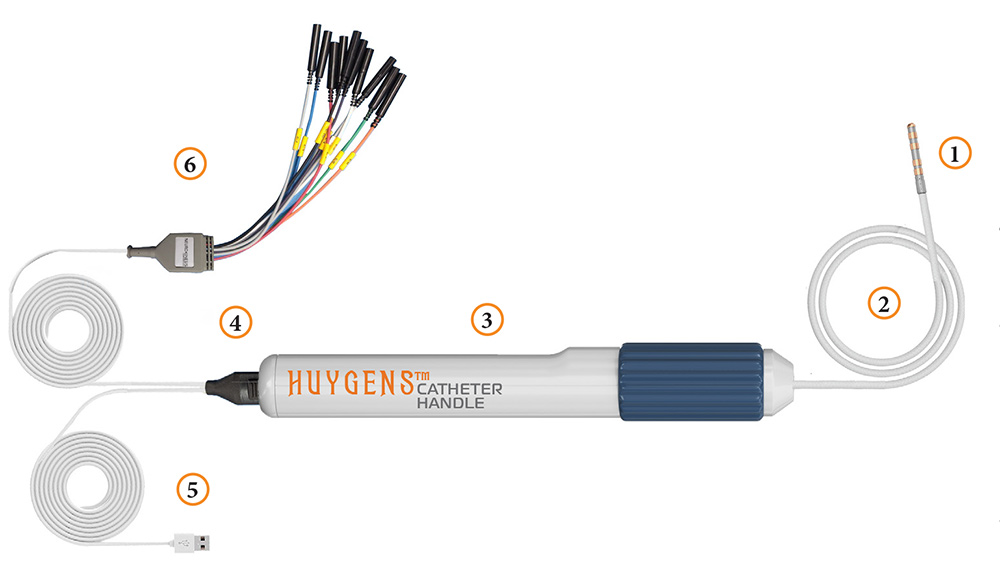
The completed design for the Huygens™ Catheter is shown here including: 1) Catheter Electrode Tip and FCB, 2) Catheter Shaft, 3) Catheter Handle, 4) Communication Connector, 5) Digital Data Communication Cable, and 6) the Ensite Nav X Communication Cable.
The final design of the sheath has been made and the initial manufacturing specs have been released to SEISA Medical for review. Approval for the design is anticipated in mid-November at which point SEISA will begin to produce the first prototypes of a complete Huygens™ Catheter with first generation electronics and the operational Huygens™ Catheter Handle. Delivery of the prototypes is anticipated for early December at which point the team will begin both wet-lab testing of the Huygens™ Catheter with the EnSite NavX for trilateration validation as well as a local non-GLP animal study to validate biopotential measurement for generation of a heart map.
Intertek Safety and EMC Testing
With the prototype catheters in hand, NKC will begin Electro-Mechanical Compliance (EMC) testing both in-house and with its compliance partner, Intertek. Intertek is a global leader in providing safety and performance certification analysis for a variety of manufacturing industries including medical devices. As well as validating the required electrical safety of the Huygens™ Catheter, Intertek will be supplying the data needed for NKC to apply for its ISO 60601-1 certification. ISO 60601.1 is the technical standard that medical device electrical equipment must meet to be considered safe and effective before they go to market. This study is scheduled to begin in the first week of December with results delivered by the end of the month. Confirmation of the device’s safety to meet the ISO standard will clear the path for the pre-clinical animal study to begin in Israel (see “Technion Animal Study” below)
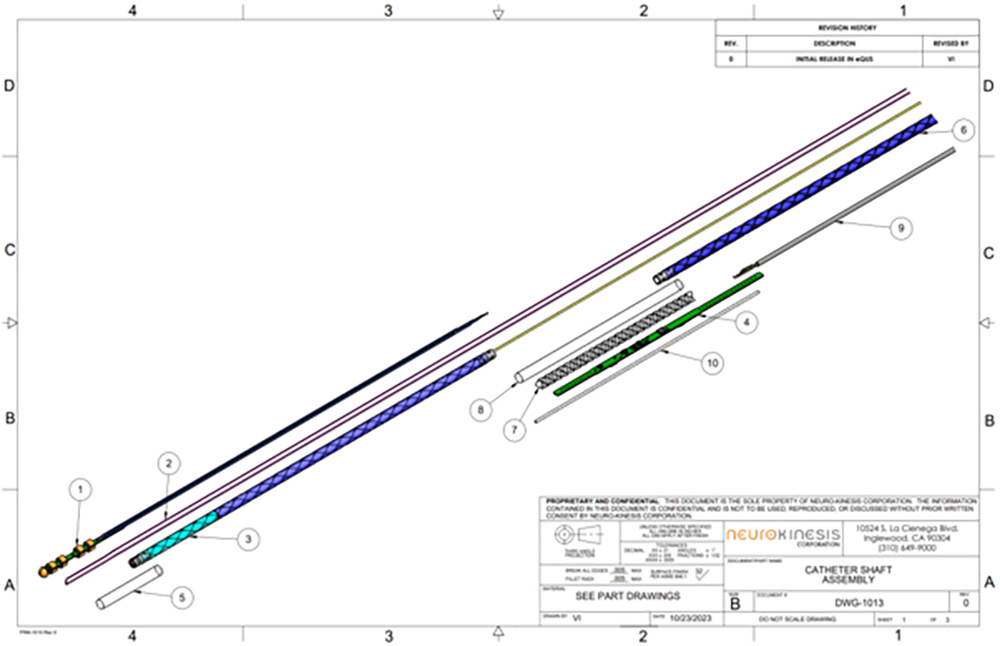
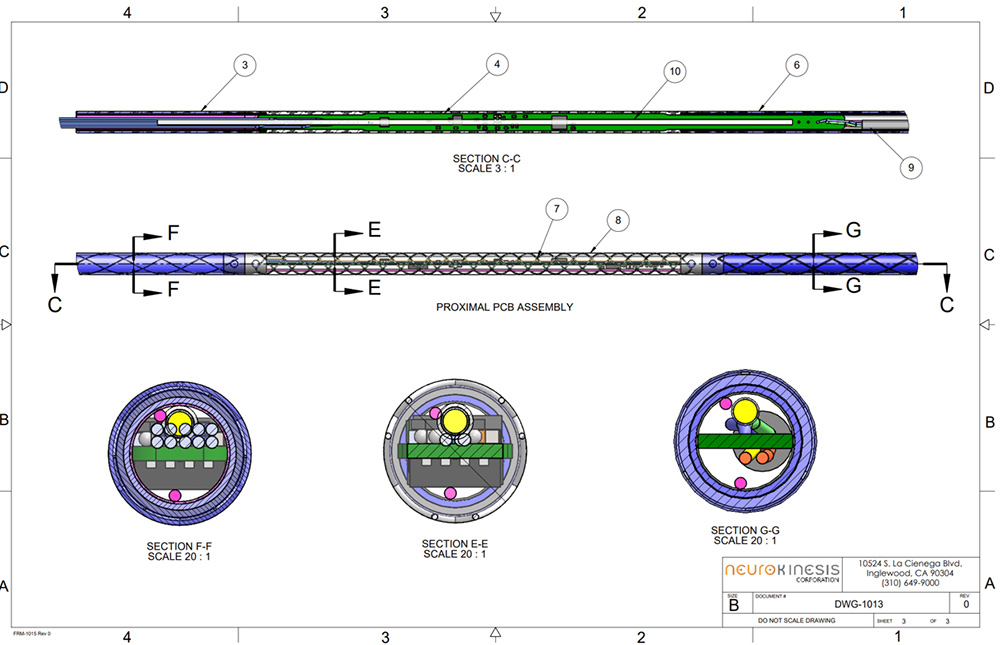
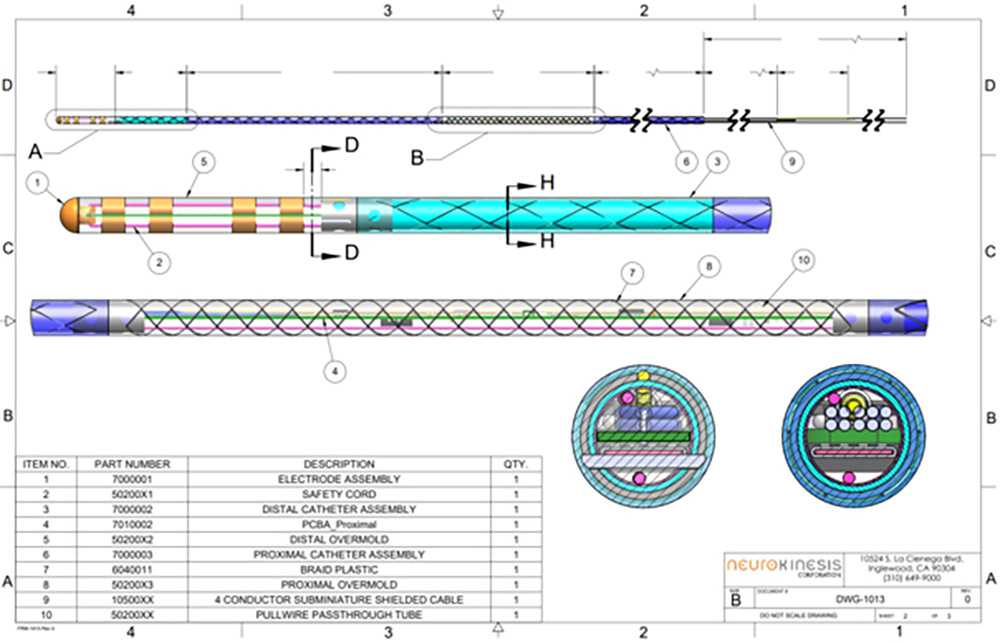
The images above show the final engineering design for the Huygens™ Catheter Shaft.
PROTEUS ROBOTIC ARM AND HUYGENS™ CATHETER HANDLE DEVELOPMENT
Advancement of NKC’s next generation of its catheter navigation system continues on track to meet the development benchmarks established last year. The Proteus Robotic Arm™ represents over 20 years of R&D in advanced robotic-assisted catheter guidance. Beginning with the world’s first magnetically controlled system CGCI in 2004 and evolving into the Proteus™ One Robotic Arm in 2020 which reduced the system from a 9-ton operating suite to a portable shoe-box-sized plug-and-play system. The Proteus™ One proved the company’s ability to meet the changing economic and medical efficacy needs of the modern EP physician in miniaturizing all the electro-mechanical features in both operating room footprint and ease-of-use into this next-gen platform.

Josh Shachar works with Tim Urick to refine the different control modes of the KUKA robot.
The Proteus™ Two represents the next phase of development for the technology as the team works to integrate robotic control and artificial intelligence (AI) to bring both real-time Human-in-the-loop (HITL) capabilities and autonomous robotic catheter mapping navigation to a reality.
The KUKA Robot Integration
In the previous report, NKC announced its acquisition of the KUKA LBR Medical Robot to serve as the primary articulated robotic arm for the Proteus™ Robotic Arm during the next phase of the technology development. The KUKA LBR robot has become a proven technology for a variety of medical procedures including precision orthopedic operations, imaging diagnostics such as comprehensive ultrasound scans, and various minimally invasive surgeries such as laser surgery, radiation knife operations, and particle-based radiotherapy.
The Proteus™ Robotic Arm provides 360* robotic controlled movement with precision repeatable navigation repeatability from ±0.1 mm to ±0.15 mm. The system has been approved both in the US and EU for medical use which gives NKC a major step forward in moving the technology into clinical trials.
An NKC engineer demonstrates the Cobot mode of the KUKA robot which allows the EP physician to manually move the arm into position for robotic guidance.
The engineering team is currently working on the next two milestones for the system which include:
- Design development of the Proteus™ Two Robotic Hand for the robot, and;
- Development of the operational integration of the system’s firmware and software for HITL navigation control.
Preliminary designs for the robotic hand are currently in progress to be able to allow for the easy transfer of the Huygens™ Catheter from the EP physician to the Proteus™ Robotic Arm for robotic control.

The Proteus™ Robotic Arm provides Human-In-The-Loop robotic navigation through an advanced Haptic controller
The team anticipates having initial design schematics for the robotic hand in place by late October and will then hand off construction of the first prototypes to a fabrication partner to be determined.

The image shows an engineer working on the design of the interface adapter that will allow the Huygens™ FCB to be integrated to the benchtop test board for in-house signal capture validation testing.
Tim became a preferred referral consultant for the Proteus™ Robotic Arm when the KUKA engineering team came out to see a robotic application he was working on for one of their clients. The company was having an issue with getting the robot to move past a singularity issue for their application. In robotics the singularity issue occurs when a six-axis robotics’ end effector, the hand or gripper, is required to move in Cartesian space in a way in which the calculations that the robot has to make to enable this movement cannot be resolved and so the movement becomes impossible. The KUKA team showed the client a possible solution that avoided the singularity and then Tim stepped in and showed a routine that just went right past the singularity problem in a much more smooth and elegant manner. The KUKA team was so impressed that Tim is now consulting with their clients around the world.
With Tim’s help, the team has already completed the ability to shift the robotic arm from its operational mode to its collaborative robot (COBOT) mode. In operational mode, the robot’s movement is controlled by external navigation input and the system’s built in safety brakes prevents any unprogrammed signal movement from occurring. In COBOT mode, the robotic arm is able to be moved manually by the physician or a technician. The COBOT mode is required when moving from manual navigation to robotic navigation as the arm must be moved into position at the catheter insertion point and then the catheter hand has to be secured in place.
The team is now working on being able to easily translate navigation control for the HITL control of the catheter with the Proteus™. For this, the team has to be able to have the robot switch between gross navigational movement for rough positioning and fine grain control for mapping inside the human heart. The goal at the moment is to have full HITL navigation capabilities in place and ready for its Phase Two animal studies to be done in 2024.
The team is also working on finalizing the design of the Huygens™ Catheter Handle which includes integration of the PM Phillips Medical data connector which will allow for the catheter’s power connection, digital data communication and Ensite NavX interface to be accommodated. Finalization of the design is anticipated to be complete in mid-November at which point the company will begin housing fabrication and assembly of the twenty completed prototype catheters to be ready for the animal study.
Human In The Loop – Integrating Proteus™ Into the EP Operating Theater
The Proteus™ Robotic Arm technology has the potential to advance the EP art into a next-century evolution of EP diagnostic and therapeutic care as it represents the first convergence of advanced robotic control and AI with seamless real-time Human-In-The Loop navigational platform for both heart-mapping and ablation operation.
The images here show how the Proteus Robotic Arm is integrated into the traditional EP procedure.
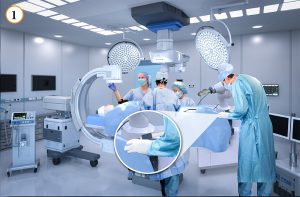 In image 1, the EP physician manually moves the Huygens™ Catheter into position through the femoral artery and into the right atrial chamber of the patient’s heart. The Proteus™ Robotic Arm is in Stow Mode with the Robotic Hand in the open position to accept the catheter handle.
In image 1, the EP physician manually moves the Huygens™ Catheter into position through the femoral artery and into the right atrial chamber of the patient’s heart. The Proteus™ Robotic Arm is in Stow Mode with the Robotic Hand in the open position to accept the catheter handle.
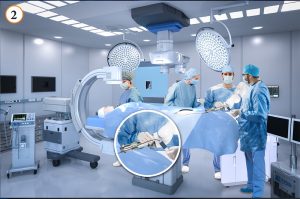 In image 2, once the catheter is in position, the Proteus™ Robotic Arm is put in COBOT Mode where is can be manually moved into operational position in order for the Huygens™ Catheter handle to inserted.
In image 2, once the catheter is in position, the Proteus™ Robotic Arm is put in COBOT Mode where is can be manually moved into operational position in order for the Huygens™ Catheter handle to inserted.
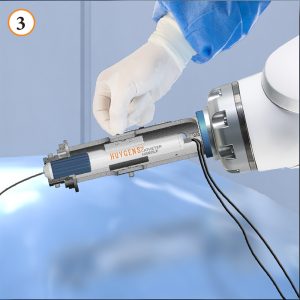 In image 3, the Huygens™ Catheter Handle is secured into the gripper of the Proteus™ Robotic Hand. The catheter handle clamp is secured and the rotating clamp is engaged with the deflection ring.
In image 3, the Huygens™ Catheter Handle is secured into the gripper of the Proteus™ Robotic Hand. The catheter handle clamp is secured and the rotating clamp is engaged with the deflection ring.
In image 4, the EP physician moves to the navigation control station where they can perform both human-in-the-loop movement of the catheter as well as manage both the biopotential and bioconductivity measurements for the creation of the high-resolution heartmap. In the future, NKC is working to add the capability of the Proteus™ to handle advanced navigational routines such as grid mapping and automatic return-to-point ablation procedures.

CLICK THE LINK BELOW TO SEE AN ANIMATION OF THE PROTEUS ROBOTIC HAND IN ACTION
TECHNION ANIMAL STUDY

The protocols for the Animal Study at Technion have been finalized and approved.
The study will look at ten subject cases. Each subject will undergo electroanatomic mapping using a commercially available steerable electrophysiology mapping catheter and the resultant map will be stored and displayed on a standard 3-D mapping system such as the NavX.
The test subjects will then undergo either EGM acquisition with the Huygens™ Catheter or EGM acquisition using a conventional known electrode catheter. The subjects will be randomized to meet the blind study protocols of the trial. These procedures will be followed by a pre-determined 30-minute observation period to ascertain that no adverse events occurred because of the Huygens™ Catheter device. Continuous intracardiac echocardiogram imaging will be used, and the pericardial space will be inspected every five minutes to confirm absence of procedure-related pericardial fluid. Both blood pressure and ECG will be monitored closely during the procedure to assure that there are no vascular complications.
Once completed, the data from the primary and secondary efficacy endpoints will be compiled and evaluated. The company anticipates it will be able to demonstrate that the Huygens™ Catheter meets or exceeds the standards of current commercially available mapping catheters and that is does so safely and is ready for FDA evaluation of the catheter for a Humanitarian Use Device (HUD) with a Humanitarian Device Exemption (HDE) which will allow the company to contemplate doing its first human clinical trails addressing patients with arrhythmia issues related to low-voltage scarring in the heart. The company also hopes the data will be sufficient for submission of the for CE Mark approval in the EU.
The trial is anticipated to take 20 weeks to complete and prepare the data for regulatory approval submission by the summer of 2024.

The images below show the state-of-the-art-animal-research capabilities at the Technion Institute in Israel. NKC’s first pre-clinical animal study of the Huygens™ Catheter begins in 2024.



SAFE HARBOR DECLARATION
THE STATEMENTS, PROJECTIONS AND ESTIMATES OF FUTURE PERFORMANCE OF THE COMPANY OR VARIOUS ELEMENTS OF THE COMPANY’S BUSINESS CONTAINED IN THIS MEMORANDUM THAT ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD EXPECT THAT ANTICIPATED EVENTS AND CIRCUMSTANCES MAY NOT OCCUR, THAT UNANTICIPATED EVENTS AND CIRCUMSTANCES WILL OCCUR, AND THAT ACTUAL RESULTS WILL LIKELY VARY FROM THE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD BE AWARE THAT A NUMBER OF FACTORS COULD CAUSE THE FORWARD-LOOKING STATEMENTS OR PROJECTIONS CONTAINED IN THIS MEMORANDUM OR OTHERWISE MADE BY OR ON BEHALF OF THE COMPANY TO BE INCORRECT OR TO DIFFER MATERIALLY FROM ACTUAL RESULTS. SUCH FACTORS MAY INCLUDE, WITHOUT LIMITATION, (i) THE ABILITY OF THE COMPANY TO COMPLETE THE DEVELOPMENT OF ITS PRODUCTS IN A TIMELY MANNER, (ii) THE DEMAND FOR AND TIMING OF DEMAND FOR SUCH PRODUCTS, (iii) COMPETITION FROM OTHER PRODUCTS AND COMPANIES, (iv) THE RESULTS OF THE COMPANY’S SAFETY AND EFFICACY STUDIES, (v) THE RESULTS OF THE REGULATORY APPROVAL PROCESS, (vi) THE COMPANY’S SALES AND MARKETING CAPABILITIES, (vii) THE COMPANY’S ABILITY TO SELL ITS PRODUCTS PROFITABLY, (viii) THE ABILITY OF THE COMPANY’S THIRD-PARTY SUPPLIERS TO PROVIDE PRODUCTS AND SERVICES IN A RELIABLE MANNER; (ix) AVAILABILITY OF ADEQUATE DEBT AND EQUITY FINANCING, AND (x) GENERAL BUSINESS AND ECONOMIC CONDITIONS. THESE IMPORTANT FACTORS AND CERTAIN OTHER FACTORS THAT MIGHT AFFECT THE COMPANY’S FINANCIAL AND BUSINESS RESULTS ARE DISCUSSED IN THIS MEMORANDUM UNDER “RISK FACTORS.” THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL BE ABLE TO ANTICIPATE, RESPOND TO OR ADAPT TO CHANGES IN ANY FACTORS AFFECTING THE COMPANY’S BUSINESS AND FINANCIAL RESULTS.

