FOR IMMEDIATE RELEASE
NKC Announces Upcoming Animal Study for Clinical Validation of its Huygens™ – Proteus™ Robotic Arm Surgical Platform
DATELINE: August 30, 2022, Los Angeles, CA
Neuro-Kinesis, Corp. (NKC), a medical technology company focused on developing next-generation surgical tools that utilize advanced biosensor technologies combined with cutting-edge AI and robotics, has announced the plans for its upcomming animal study for its new Huygens™ Catheter. The study is designed to demonstrate the ability of the Huygens™ Catheter to generate clinical data that is superior to the existing art, thereby demonstrating improved clinical indices in electrophysiological therapeutic procedures.
Multi-center trials to commence Q1 2023 at Technion and Sandia
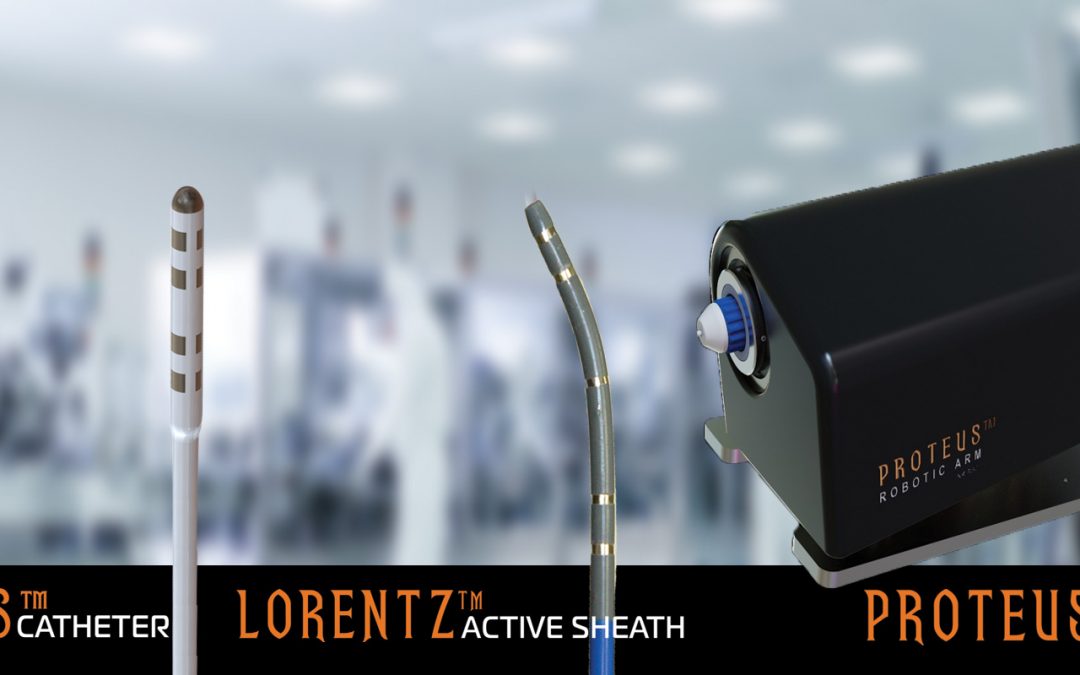
Neuro-Kinesis Corp. (NKC) has submitted an Application for an Institutional Animal Care and Use Committee Approval (IACUC) for its upcoming animal studies of the Huygens™ – Proteus™ Robotic Arm Surgical Platform. An agreement has been signed with the Technion Institute in Israel to begin the animal study validation of NKC’s surgical platform in Q1 2023. The platform includes the Huygens™ Catheter, the Lorentz™ Active Sheath, and the Proteus™ Robotic Arm which have been in development over the last several years.
The study will be headed by NKC Chief Electrophysiology Officer, Dr. Eli S. Gang, MD (Clinical Professor of Medicine,

Dr. Eli Gang
Geffen School of Medicine at UCLA, Cedars Sinai Medical Center). The study will be performed under the guidance of Principal Investigator Dr. Rona Shofty, DVM, PhD, DLAM.
The study as shown will demonstrate the ability of the Huygens™ Catheter to generate clinical data that is superior to the existing art, thereby demonstrating improved clinical indices in electrophysiological therapeutic procedures. Specifically, the study is designed to demonstrate the ability of the Huygens™ Catheter to perform detailed mapping and target acquisition procedures in all four chambers of the test subject’s heart. Success in this study will constitute proof-of-design adequacy and equipment safety in reaching the efficacy and safety goals established in the study protocols.
A separate agreement is being finalized with Sandia National Laboratories to perform real-time simulation studies of the system under the guidance of Dr. Darren W. Branch, PhD., and is anticipated to proceed concurrently to demonstrate the embodiments that differentiate the Huygens™ Catheter from any other catheter in the marketplace today.
System Integration to be done at NKC Headquarters

NKC Validation Lab
The lab installation will allow the NKC engineering team the ability to test prototype designs, perform quality control checks, and establish operational and test protocols while gathering the important analytical and comparative clinical data needed to further its regulatory approval strategy.
New NKC Patent Filing
 NKC has filed a new patent application developed by NKC’s CTO Josh Shachar. Titled, “The Use of Local Amplifiers and a Huygens Sensor Array in Measuring Bioelectric Signals and Clinical Applications Thereof” (PCT/US2022/030399), the patent relates to the field of electrophysiological mapping methods using the Huygens™ Catheter with its capabilities of co-measuring impedance and local native biometric signals, and employing such signals with a method that identify a “phase singularity” within the electroanatomical space as well as its dynamics.
NKC has filed a new patent application developed by NKC’s CTO Josh Shachar. Titled, “The Use of Local Amplifiers and a Huygens Sensor Array in Measuring Bioelectric Signals and Clinical Applications Thereof” (PCT/US2022/030399), the patent relates to the field of electrophysiological mapping methods using the Huygens™ Catheter with its capabilities of co-measuring impedance and local native biometric signals, and employing such signals with a method that identify a “phase singularity” within the electroanatomical space as well as its dynamics.
Clinically, this means the Huygens™ Catheter might solve one of the most fundamental problems in electrophysiology, and can then be used as a standard tool for care in treating pacing problems such as A-Fib and other disease models.
To date, NKC has filed eight patents, both domestically and internationally, describing its
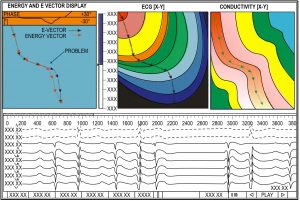
One of the advances enumerated in the patent, is the ability of the Huygens™ Catheter to accurately identify and measure phase singularities in order to provide a more accurate representation of the bioelectrical activity in the heart chamber.
General Integration of NKC Platform with Abbott/St. Jude EnSite NavX System
Abbott/St. Jude EnSite NavX EP Mapping System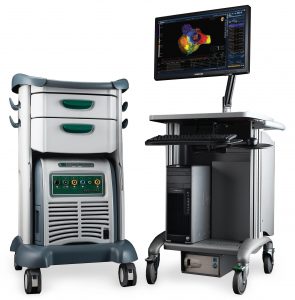
From 9 Ton to 9Kg
The Huygens™ – Proteus™ Surgical Platform represents two decades of work in advancing robotic catheter guidance from its original 9-ton flagship CGCI (Catheter Guidance Control & Imaging) Robotic System down to the 9kg device it is today.
CGCI was one of the first catheter guidance systems that used magnetic fields to navigate a specially designed catheter

CTO and Inventor Josh Shachar showing comparison of the 9kg Proteus™ Robotic Arm next to the 9 ton CGCI..
The advances made with CGCI, and its early-development prototype, the MOSFET Catheter counterpart, have paved the way for the Huygens™ Catheter and the Proteus™ Robotic Arm, reducing the technology that required a dedicated operating suite into a portable, plug-and-play system that can be deployed into any existing EP operating room while delivering detection sensitivity, mapping resolutions and AI enhanced guidance that can dramatically change the face of EP cardiac diagnostics and treatment.
Addition of Seasoned Regulatory Expertise to NKC Team
NKC has contracted two new team members to navigate the FDA regulatory pathway for product approval of the Huygens™ Catheter and the Proteus™ Robotic Arm technologies: Prof. Elaine Duncan (Paladin Medical Inc.) and Dr. Jaap Laufer MD (Emergo Group Inc. Both of these highly qualified individuals bring to the table decades of experience in successfully guiding groundbreaking medical technologies to market.
Professor Duncan is the founder and president of Paladin Medical®, Inc. and is a certified regulatory affairs professional with a master’s degree in engineering. She holds an appointment as an Adjunct Professor of Biomedical Engineering in the F. Joseph Halcomb III, M.D. Department of Biomedical Engineering at the University of Kentucky and is a recognized leader in regulatory/clinical strategies for new medical technology development.
Dr. Laufer has over 30 years of regulatory and clinical experience specializing in implant, high-risk device and combination product submissions, FDA QSR compliance, and clinical study submissions and compliance. He has held corporate positions in Regulatory and Clinical Affairs for the Switzerland–based Lipomatrix, Pfizer Hospital Products Europe, and global giant Abbott Laboratories.
Together, along with Lead Regulatory Consultant, Susan Alpert MD, who has 30 years of experience and was the former Senior Vice President of Global Regulatory Affairs, at Medtronic, and Quality Engineer Elissa Salceda, who has been hands-on in the development of the Huygens™ – Proteus™ Robot Arm Surgical Platform, the NKC regulatory team is on a solid path forward to achieving its first major commercialization milestone, FDA approval. This milestone will allow entry of the NKC technology into the U.S. marketplace. This will also provide benchmark clearance for the European CE Mark.

Prof. Elaine Duncan

Dr. Jaap Laufer MD

Dr. Susan Alpert

Elissa Salceda
Anacaz Group to Develop Digital Backend for NKC Clinical Data Management

NKC is in negotiations with the Anacaz Group to support the development of cloud-based operations and ensure the security and safety of the preserved patient data acquired with the Huygens™ – Proteus™ Surgical Platform. This data will allow physicians to review the exact conditions and events recorded during a procedure including placement of ablative lesions, rotor locations, and the complete replay of actions taken by the surgeon. The establishment of this dedicated secure cloud platform will provide the ability for patient information and treatment to be accessible to the patient-designated healthcare providers as well as creating a growing database of information to be available for expanding the knowledge base of cardio-disease treatment for EP physicians everywhere. Creating this secure central share-point is in alignment with NKC’s vision of helping to improve the EP art, and to democratize medicine for everyone.

Robert Abrams
The Anacaz Group is the recognized leader in high-speed L4-L7 packet processing. The company was founded by Robert Abrams who has 30 years of experience and was central in providing many high-tech innovations including the Veritas High-Performance File System, Cisco Catalyst 5000, Cisco PIX Firewall, SonicWALL Cyber Security Solutions, Apple iMac Pro and MBP, and the AMDI Labs AutoLab Antibody line of test solutions. He has worked with major tech industry players such as Cisco, Apple, Fujitsu, Sony, Samsung, Broadcom, as well as many other Fortune 500 companies.