
by teamneuro-kinesis | Dec 13, 2011 | TImeline
In December of 2011, The CGCI-II platform was granted its CE mark designation. The Conformitè Europëenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area (EEA). A CE Mark...

by teamneuro-kinesis | Oct 20, 2011 | News
A CGCI suite has been installed at the Yonsei University Severance Hospital in Seoul, South Korea. This installation represents the first such in the Asia market. Clinical trials will be conducted at the hospital using CGCI for mapping and ablation procedures...

by teamneuro-kinesis | Oct 13, 2011 | TImeline
A second CGCI EP Suite was installed at Yonsei University Hospital in Seoul, Korea. The installation was completed in October of 2011 and was placed under the direction of Dr. Huy Nam Pak, Director of Electrophysiology for the hospital, to begin the process of gaining...

by teamneuro-kinesis | Oct 13, 2011 | TImeline
The CGCI was given IEC 60601-1 Second Edition Certification from the International Electrotechnical Commission (ICE). ICE is an international standards organization that prepares and publishes international standards for all electrical, electronic and related...
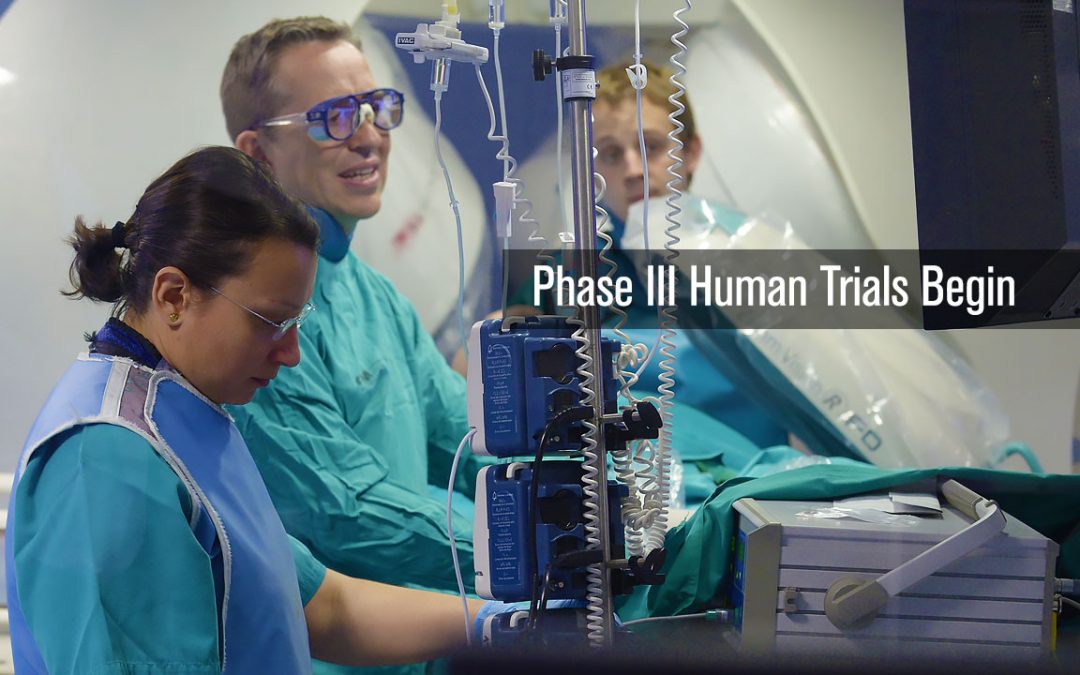
by teamneuro-kinesis | Sep 13, 2011 | TImeline
Building on the success of the first two human trails of the CGCI platform in Madrid, Spain, a 54 patient Phase III Trial is conducted to perform standard atrial as well as paroxysmal atrial ablations. The goal of the study was to perform a series of atrial ablation...






