CGCI
Neuro-Kinesis’ Catheter Guidance Control and Imaging system (CGCI) represents a quantum leap forward in robotically-controlled catheter guidance systems using lensed magnetic arrays to provide precision navigation of a catheter tip to a targeted location for imaging, ablation, or other procedures, with the singular advantage of being to provide repeat return to the site with the push of a button.
Unique to the CGCI platform is its scalable controlled magnetic lensing, precision control of the movement of an
object in three-dimensional space using variable field magnetics, and the ability to do this with lower levels of input power, greater reclamation, and regeneration of the magnetic field wave – all at a minimum risk exposure to the patient. What was thought impossible just 20 years ago, Neuro-Kinesis has made a reality that has the potential to benefit the lives of countless patients where this technology can be more effective, more cost-efficient, and more reliable in order to provide a better solution.
Creating New Frontiers for Catheter-Based Treatments
CGCI was created to provide physicians a robotic catheterization control systems for minimally invasive procedures. Our system and proprietary designed catheters, tailor-made for specific treatment applications, promise a potential co-venturing Strategics where precision guidance systems can be used to exponentially enhance the delivery and therefore effectiveness of their treatment catheter-based treatment procedures.
With CGCI, Neuro-Kinesis is positioning itself as a global leader in the development of advanced medical technologies utilizing robotic control and electromagnetic navigation methodologies that increase the efficacy and efficiency of physicians performing minimally invasive procedures in areas such as cardiology, gastroenterology, neurology and gynecology, and more. The multiple benefits of the CGCI electromagnetic guidance and control systems and advanced catheter toolsets are expected to dramatically reduce the cost of healthcare by bringing safer, more effective, and efficient treatment methods to an ever-increasing number of patients.
features
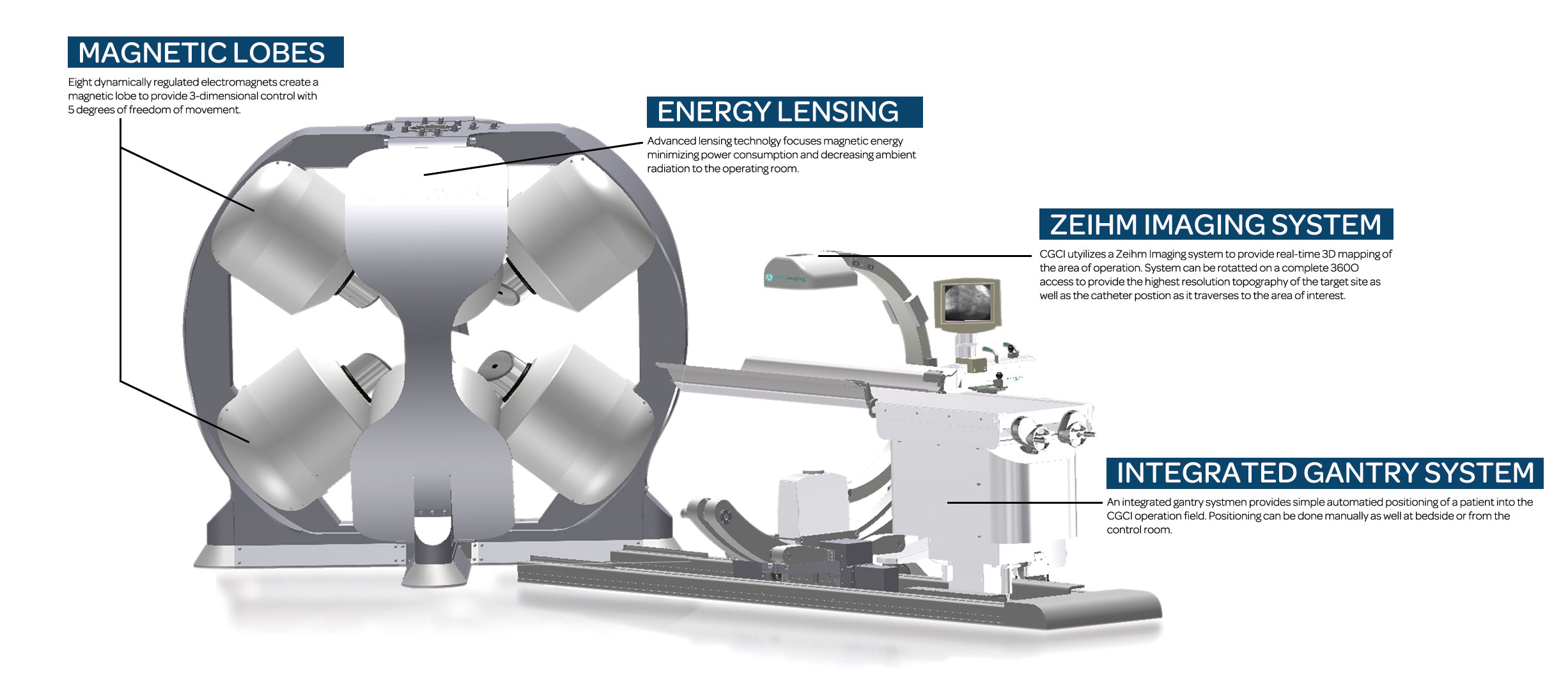
- eight electromagnet lobes arranged in a unique configuration to provide precision intelligent guidance of a magnetically-tipped catheter in all 3 dimensions of space.
- advanced magnetic energy lensing provides precision control of magnetic forces to the catheter while minimizing any leakage of energy into the surrounding operating room resulting in greater safety to the patient and the operator.
- the CGCI Symphony™ Interface integrates all controls into a unified console that allows the physician complete access to all telemetry, patient diagnostics, and navigation systems.
advanced guidance and diagnostics systems. - intuitive navigation controls provide ergonomic ease in controlling the catheter in all 3 dimensions of access while the advanced 3-D mapping systems provide high-resolution imagery of the operating area.
- continuous system monitoring provides real-time feedback on the operational status for every component of the CGCI platform.
CGCI SYMPHONY CONTROL SUITE
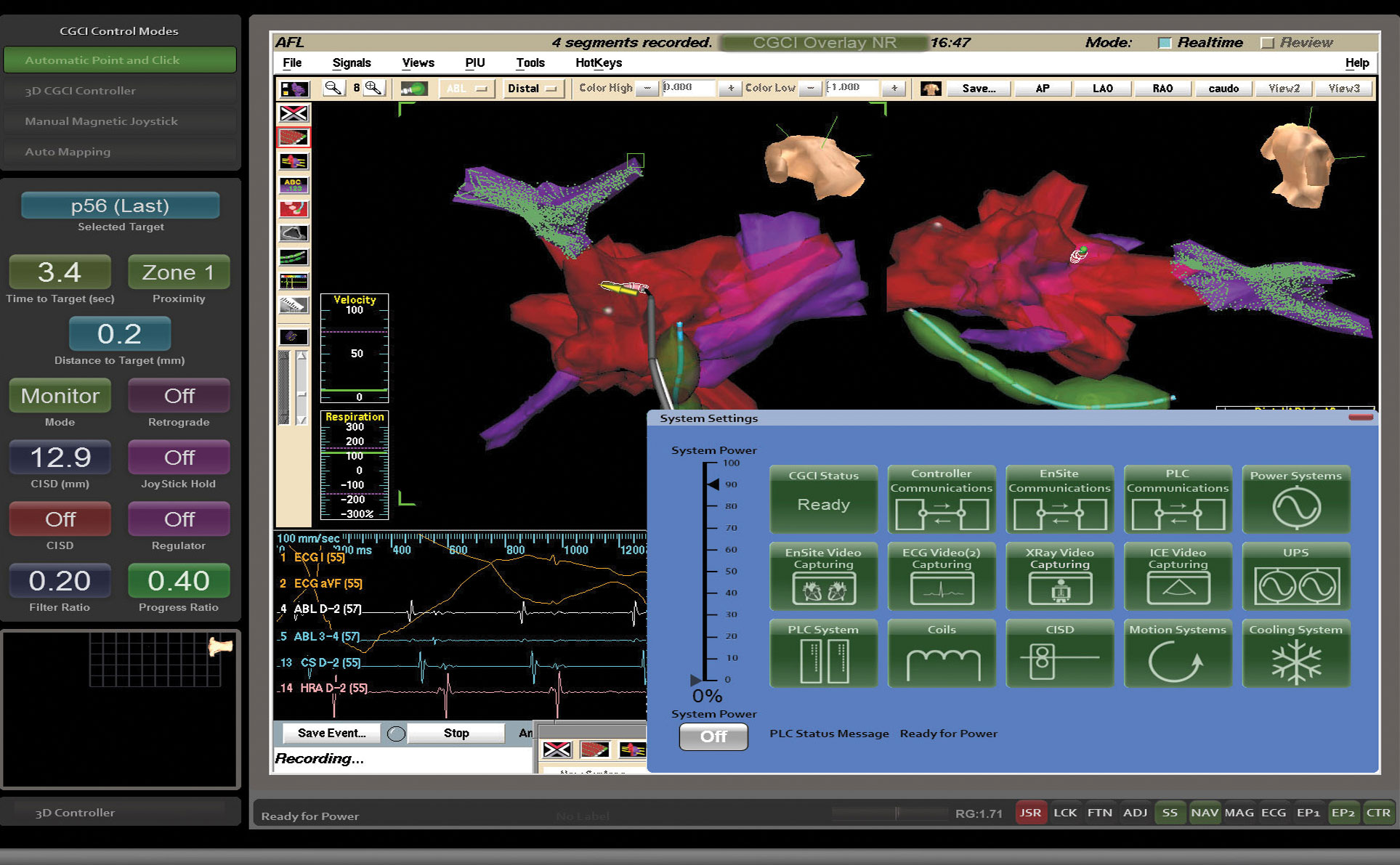
The CGCI Symphony Operating Suite is a complete self-contained control system that allows a physician to easily operate all aspects of the catheter guidance, environmental mapping, and procedure operation such as ablation or biopsy while being able to have complete visual tracking of a variety of patients statistics including, ECG, EKG, system vitals and imaging readouts from X-Ray and Fluoroscopy.
The single CGCI 3D console displays real-time Ensite mapping system readouts, unification with fluoroscopy, ECG System, and intracardiac echocardiography (ICE) display.
The system is controlled via remote control with integrated management of all EP systems including the EP recording system, 3-D electroanatomical system, electrical stimulator, fluoroscopy system, and intracardiac echocardiography. A single operator can determine the patient’s arrhythmia mechanism as well as control the ablation process, removing the need to provide instruction to different people managing various EP systems.
Catheter control can be done via manual magnetic joystick-control offering nearly instantaneous catheter tip movement and response within a cardiac chamber or by using the CGCI logic routines which can plan a path to reach the targeted location, determine the optimal contact point and then guide the catheter tip until it makes firm and continuous tissue contact in anticipation of the ablation procedure.
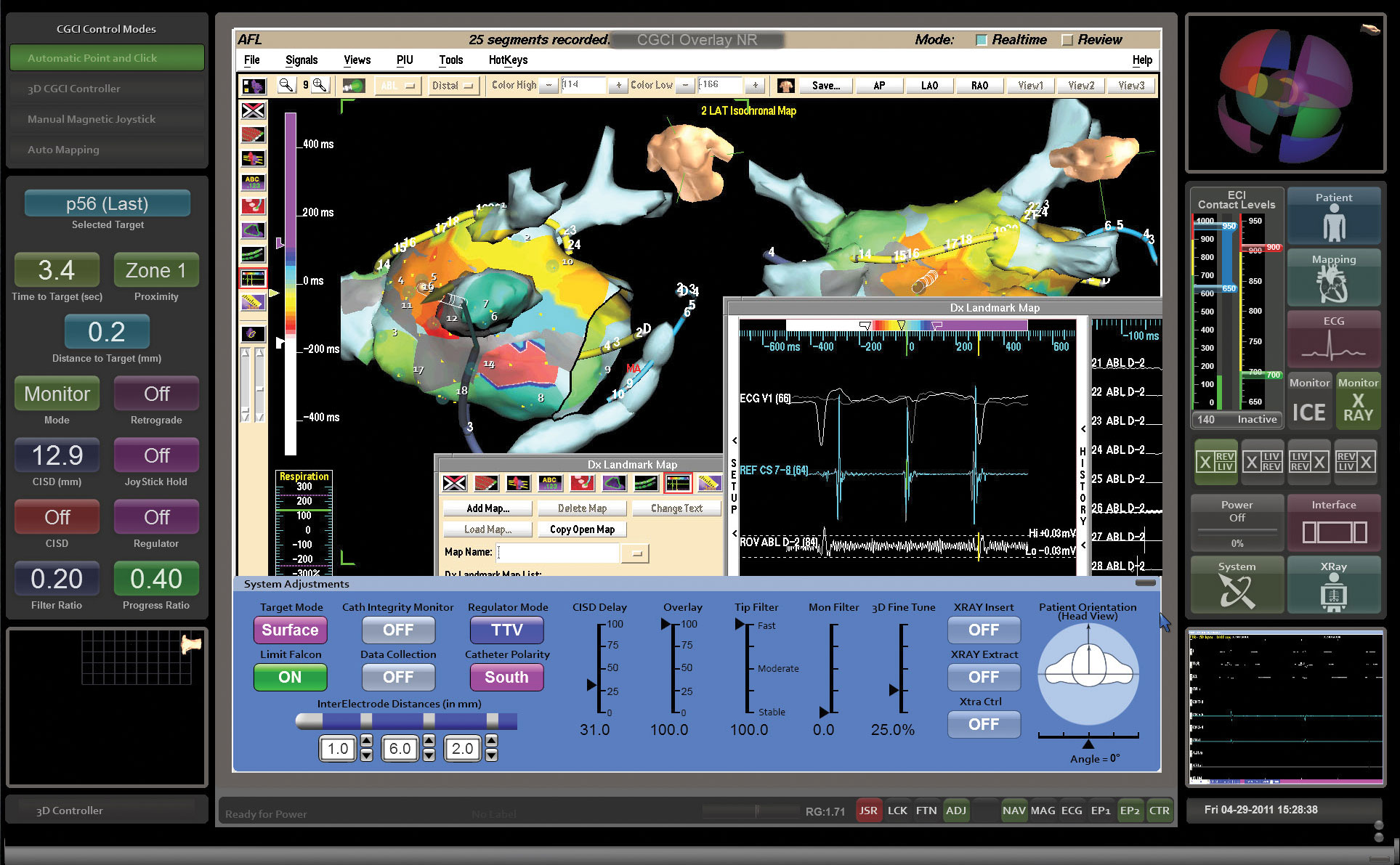
INTUITIVE NAVIGATION CONTROL
The intelligent user interface gives the physician complete navigation control of the entire platform for true automaticity both in mapping and ablation procedures.
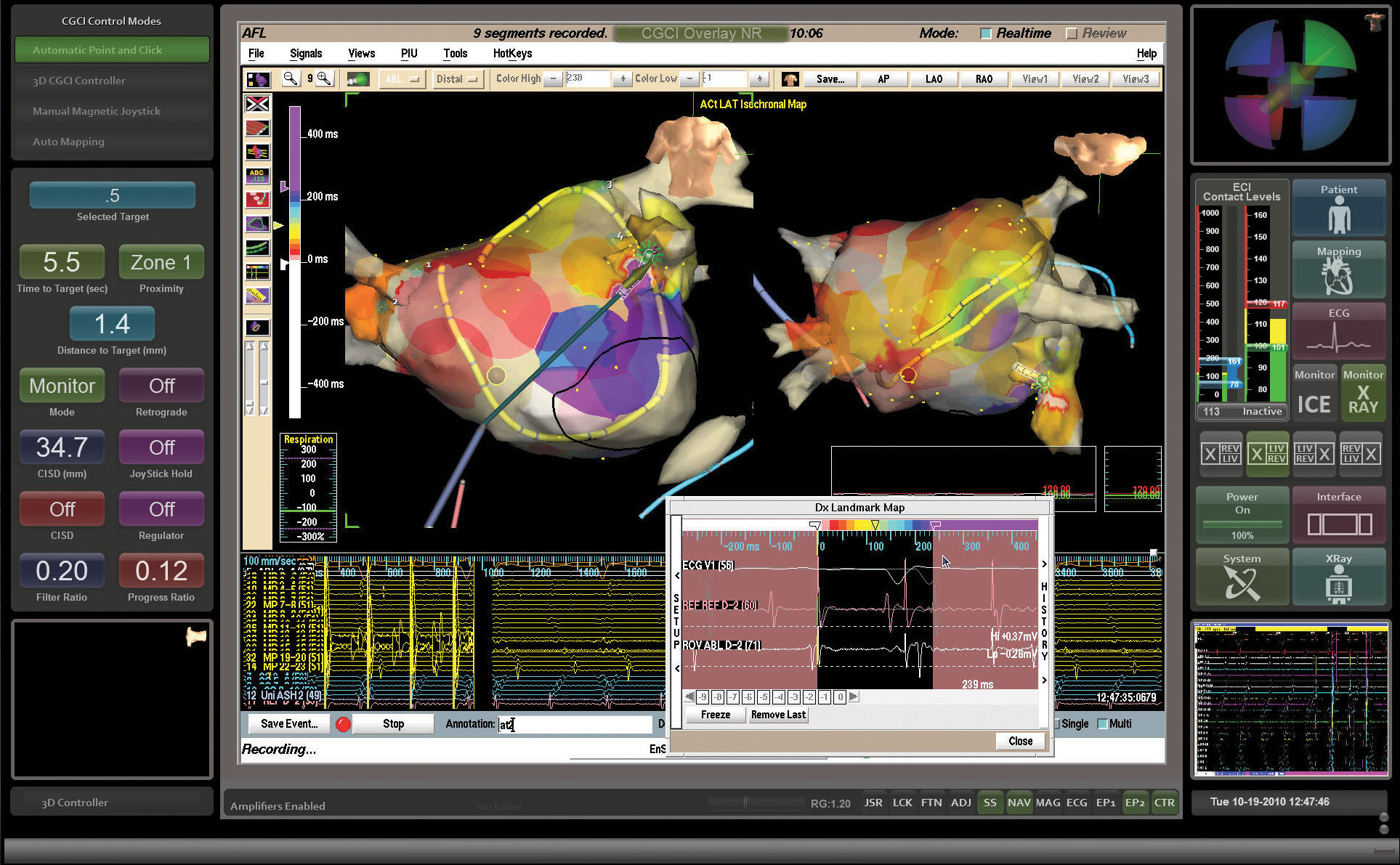
ATRIAL ACTIVATION MAPPING
Atrial activation mapping technology allows the physician to accurately view the procedure environment.
ADVANCED SYSTEM MONITORING
Continuous system monitoring provides real-time feedback on the operational status for every component of the CGCI platform.
CGCI TImeline
The CGCI Platform is the result of over 25 years of research, testing, and stringent trials to bring it to its current pre-market commercialization readiness. Here are some of the important milestones that have been achieved along the way.

Third CGCI Suite Constructed
A CGCI-II EP Suite is installed in the Na Homolce Hospital in Prague, Czech Republic. Under the direction of Dr. Petr Neuzil, Head of Cardiology for the Hospital, and Dr. Vivek Reddy, Professor of Medicine and Director of Cardiac Arrhythmia Service at Mount Sinai...

CE Achieved
In December of 2011, The CGCI-II platform was granted its CE mark designation. The Conformitè Europëenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area (EEA). A CE Mark...

Second CGCI Suite Completed
A second CGCI EP Suite was installed at Yonsei University Hospital in Seoul, Korea. The installation was completed in October of 2011 and was placed under the direction of Dr. Huy Nam Pak, Director of Electrophysiology for the hospital, to begin the process of gaining...

IEC Certification Achieved
The CGCI was given IEC 60601-1 Second Edition Certification from the International Electrotechnical Commission (ICE). ICE is an international standards organization that prepares and publishes international standards for all electrical, electronic and related...
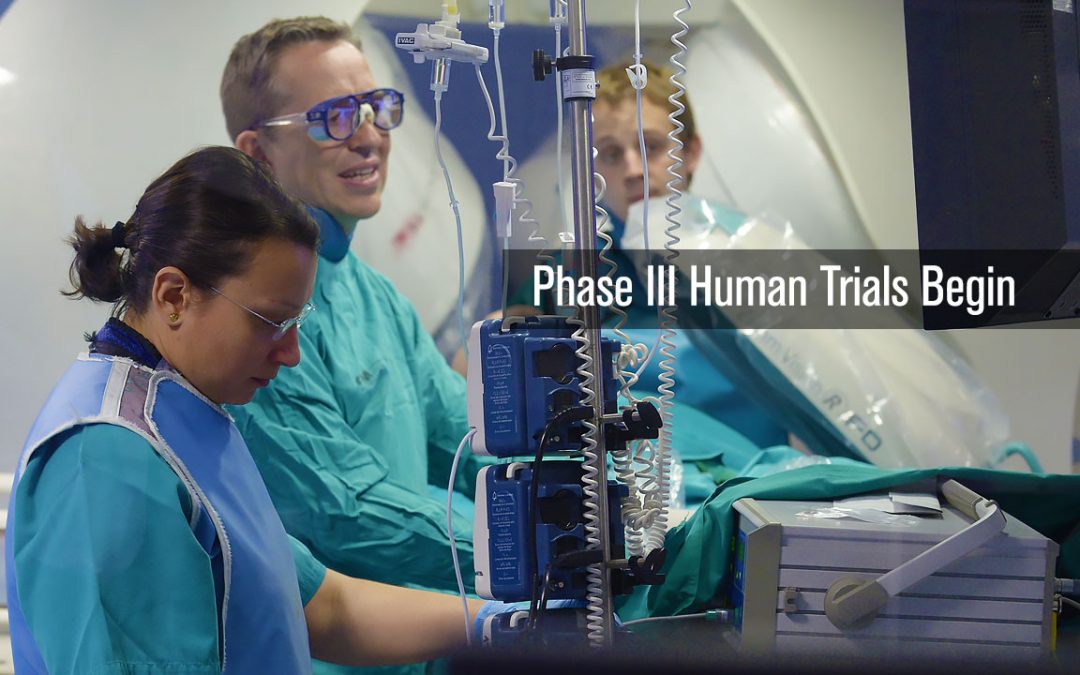
Phase III Human Trials Begin
Building on the success of the first two human trails of the CGCI platform in Madrid, Spain, a 54 patient Phase III Trial is conducted to perform standard atrial as well as paroxysmal atrial ablations. The goal of the study was to perform a series of atrial ablation...

Eleven More Patents Filed
An additional eleven patents are filed over the course of the year relating to the apparatus and method for the Lorentz-Active sheath display and control of surgical tools, creating a high-resolution map of electrical and mechanical properties of the heart, magnetic...

ISO-13485 Certification Achieved
In another step forward in completing all pre-market regulatory compliance needs, the company received its ISO-13485 Certification. The certification specifies requirements for a quality management system where an organization needs to demonstrate its ability to...

Human Trails Commence In Madrid
A full CGCI Suite was installed at the University Hospital of La Paz in Madrid, Spain in order to begin a 20 patient human trail. Under the direction of noted cardiologist and Principal Investigator, Dr. Jose Merino who is the Director of the Arrhythmia and...

CGCI-II Constructed
The first full-scale prototype of the CGCI platform is completed. The unit utilizes eight magnetic lobes, each containing a twenty-four-inch coil capable of producing a magnetic field of 1900 Gauss. The unit stands seven feet tall and has a floor weight of 20,000lbs....

UL Certification Granted
The CGCI platform was granted UL Certification thereby approving the device for use in the medical field. This certification certifies that the CGCI Platform meets all the stringent electrical safety standards required for both electrical safety concerns as well as...

CGCI IP Portfolio Expanded
Additional breakthroughs in the advancement of the CGCI technology platform result in nine patents being submitted relating to the apparatus and method for controlling catheter positioning and orientation, the magnetic linear actuator for deployable catheter tools,...
Nine More Patents Filed
An additional nine patients over the course of the preceding year relating to CGCI’s apparatus, method, and system for radar-assistance as well as the first patents for what would become the Optical Catheter are filed. This patent represented the first application for...
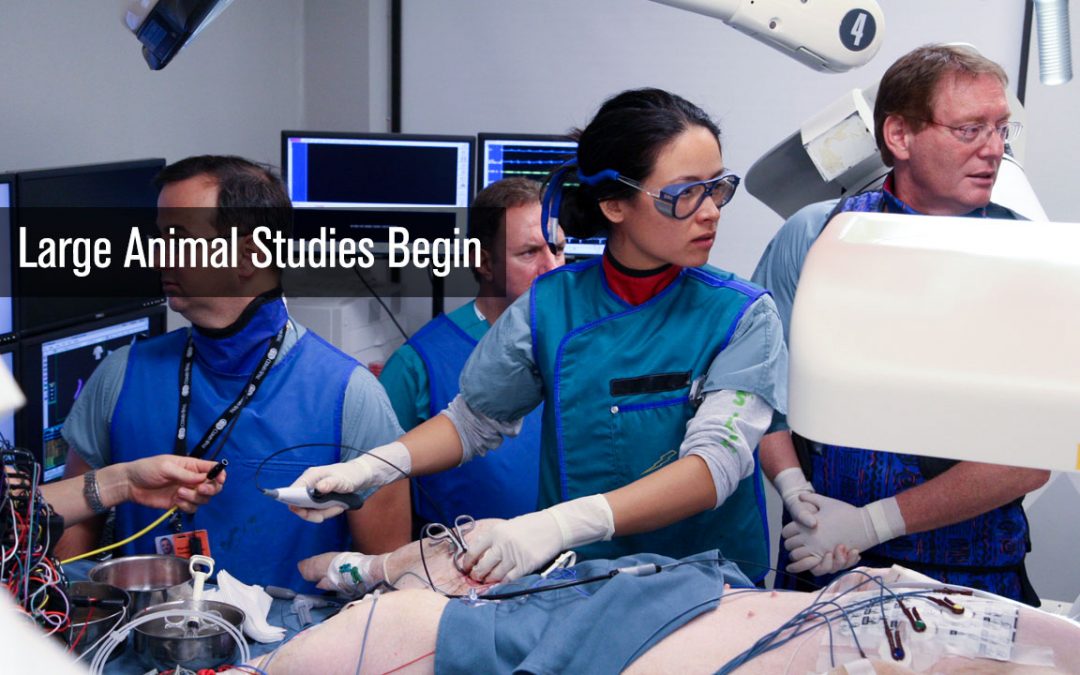
Large Animal Study Conducted
The first large animal study utilizing the CGCI-1 begins at Cedars Sinai Hospital in Los Angeles, California. The study consisted of 15 subjects undergoing a simulated AFib procedure to show the ability of CGCI to move a catheter to an identified target site, remember...

Scale Model of CGCI Built
The first fully operational prototype of CGCI is constructed. Built at a 2/3 scale, CGCI-1 featured an 8-lobe model using 18” coils and was capable of generating a magnetic field of 900 Gauss. The unit was installed at Cedars Sinai Hospital in Los Angeles, CA, and was...
Patent #11/140,475 Filed
Patent #11/140,475 is filed describing CGCI’s proprietary methodology for shaped magnetic field control. This represented a major technological step forward for the platform.
Seven More Patents Filed
An additional seven more patents are filed relating to breakthroughs in CGCI’s method and system of utilizing radar-guidance to control a catheter.
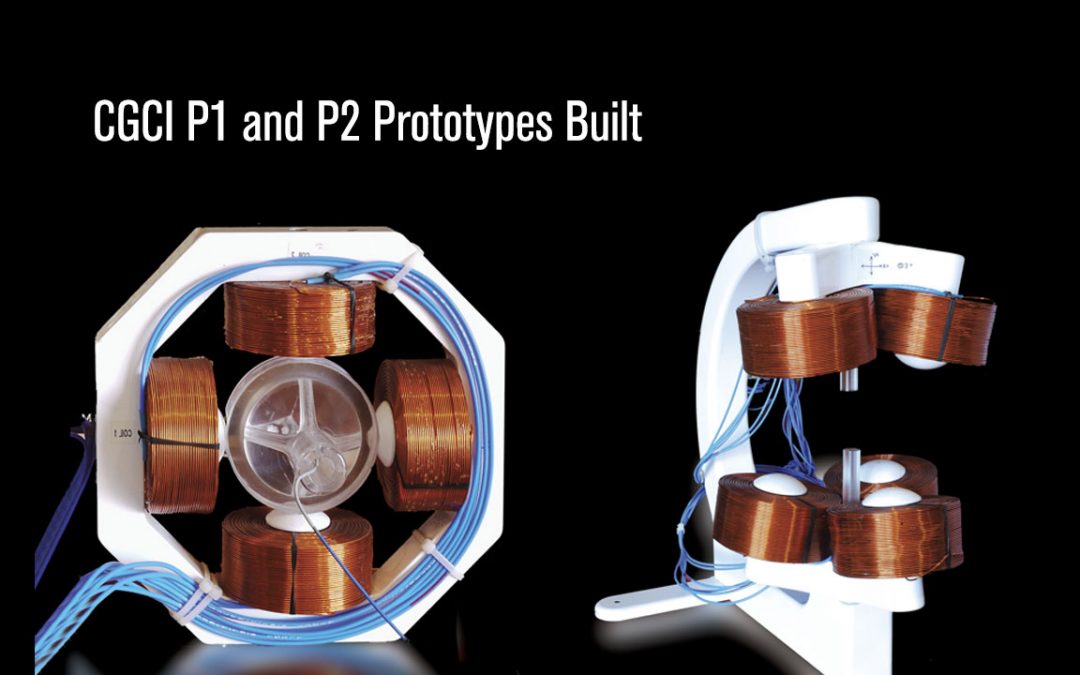
CGCI Prototypes Built
P1 and P2 Prototypes of the CGCI system are built to validate the concept and solve scalability issues. The CGCI – P1 was the first prototype to measure baseline magnetic field density “pole to pole” in a two-dimensional environment. It was four inches in size and...

Additional Nine Patents Filed
An additional nine patents are filed relating to its CGCI apparatus method as well as the system and methodfor radar-assistance.
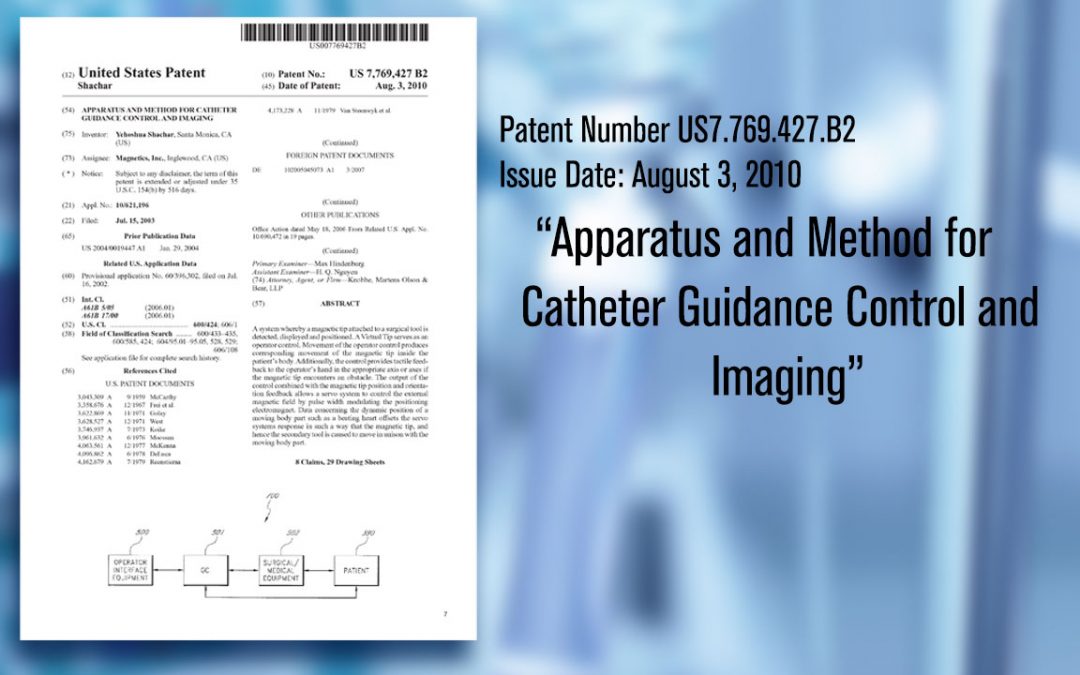
First Patent Filed
Josh Shachar files first patent for “Method and Apparatus for Catheter Guidance Control and Imaging.”
“At the core of any task is the fundamental and unassailable belief that a solution is possible.”
– Josh Shachar
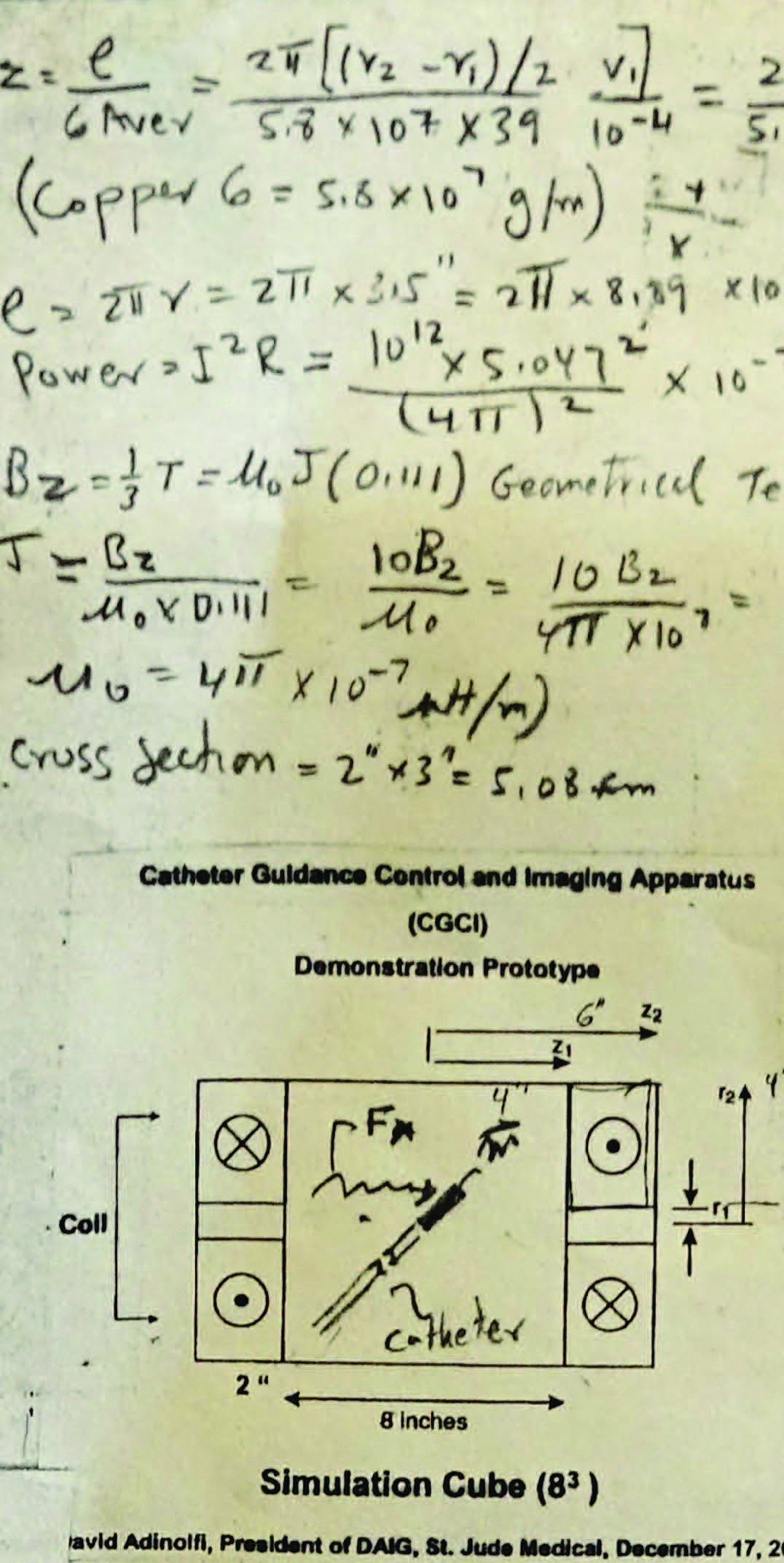
Above: Excerpt from Josh Shachar’s early notes on solving the problems associated with the scalability of a magnetic field for CGCI. As in all answers to any problem he tackles, Josh first looks to nature where the perfection of “Form and Function” always shows the best path to solving any puzzle.
A Path to Optimizing AFib Treatment
Physician-guided, catheter-based disease treatment is a growing and accepted standard for procedures such as Atrial Fibrillation (AFib) ablation, endovascular coiling, transurethral prostatectomy, and endoscopic exploration. However, these procedures come with significant risk including minimal effectiveness requiring repeat procedures, vascular injury, organ perforation, blood clotting, septic infections, stroke, and even death. Key to the effectiveness of physician-guided catheter procedures is the ability of the physician to accurately position the catheter tool “on-site” where it is needed while minimizing invasiveness to the patient’s system and optimizing the results for a positive patient outcome. In procedures where the distance from the catheter insertion point to the treatment site is short, the channel is relatively large and the target area can be easily visualized, these risks are significantly low, such as the resection of the prostate gland. When any of these parameters change, the risk increases exponentially.
One of the areas where this risk is of large concern is the treatment for AFib. To treat AFib using catheter ablation, a catheter must be guided from an insertion point in the groin or neck of a patient, through the arterial network and into a chamber of a beating heart where it must then precisely “ablate” or destroy portions of tissue in order to lessen or eliminate the recurrence of AFib. Currently, this procedure relies solely on the skilled hand of the physician manually navigating the catheter using a two-dimensional imaging visualization aid such as an X-ray or fluoroscopy. The risk potential is obvious when the environment the physician must traverse is not only three-dimensional but a moving target as well, as is the case of placing a catheter in a specific chamber of a living, beating heart and then manipulating the catheter to precisely ablate multiple sections of the heart tissue in order to correct cardiac arrhythmia.
It is this area that was foundational to the vision of developing CGCI when through a personal situation Neuro-Kinesis’ Co-founder and Chief Innovation Officer Josh Shachar pondered the possibility of creating a method to give a physician a tool that would allow them to be in harmony within the environment with which they are working instead of being a third-party observer outside of the realm in which they are trying to affect a cure. In short, was there a way to allow a surgeon to be as virtually “inside” the area of operation as they are physically “inside” an operating room? A way to allow the doctor to work in tandem with the three-dimensional living body in order to more effectively treat his patient.
Looking to his decades of experience in developing guidance systems for the U.S. Department of Defense (DOD) where he had been behind the innovation of many of our nation’s weapon system guidance technologies, Josh Shachar realized that adapting a solution to the issue of catheter guidance was simply a matter of scale and environment. He assembled his engineering team and they quickly settled on the concept of using opposing magnetic force fields as the basis for controlling an object’s position in three-dimensional space. With the filing of the first patent for their developing technology titled “Method and Apparatus for Catheter Guidance Control and Imaging”, the CGCI platform entered into developed to create the first robotic-controlled platform using magnetic force for the remote navigation to guide surgical instruments within a patient’s body.

