FOR IMMEDIATE RELEASE
NKC Releases 2023 End-of-Year Development Report on the Huygens™/ Proteus™ Technology
DATELINE: December 28, 2023, Los Angeles, CA
Neuro-KInesis, Corp. (NKC), is providing the following to share the 2023 third quarter updates on Neuro-Kinesis’ (NKC) progress in forwarding the development of its Huygens™/Proteus™ Smart Catheter and Robotic Guidance System. The Huygens™ Catheter and the Proteus™ Robotic Arm are NKC’s lead product candidates for its line of SMART Surgical Tool technologies that combine advanced bio-data acquisition with enhanced Artificial Intelligence using proprietary micro-engineering and robotic-assisted control to provide surgeons an expanded tool-set to be able to deliver better surgical outcomes for their patients.
PROTEUS ROBOTIC ARM
2023 has seen some major advancements for NKC’s robotic catheter navigation system. In the short twelve-month period, the engineering team was able to take all the electro-mechanical innovation it had achieved with the Proteus™ One Robotic Arm and transfer it to the Proteus™ II Robotic system. The Proteus II™ integrates the advancements made in medical robotics into a navigation system capable of performing the precision movements required for catheter guidance of an electrophysiological (EP) catheter inside the moving dynamic of the human heart for both anatomical mapping and cardio-ablation procedures. The Proteus™ II is the culmination of over two decades of work by NKC’s co-founder Josh Shachar in seeing his vision for advanced robotic-assisted guidance, and potentially autonomous AI-enhanced control of an EP catheter come to reality. The Proteus™ II has the potential to open a level of precision, consistency, and far more comprehensive mapping and ablation control than has ever been possible.
Robotic Arm Testing
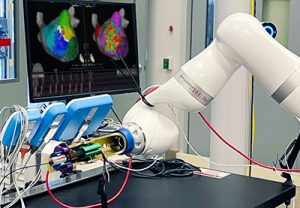
The first prototype for the Proteus™ II Robotic Arm Gripper is shown during initial testing.
As stated in the Third Quarter update, the NKC engineering team along with advanced robotic applications consultant Tim Urick have been working to adapt the base navigational software in the KUKA Medical robot for the specific needs of NKC’s robotic catheter control platform. The team has been able to successfully confirm and test the operation of the robotic arm in all three modes required in an EP procedure. These include Brake Mode, where the robot is locked into a position that cannot be affected by unwanted movement or forces, such as someone bumping into it in a critical moment, and Operational Mode,

During initial navigational testing, the Gripper operated flawlessly in performing rotational and translation movements.
where the robot can be manually moved and adjusted into position by outside physical forces. This is required once the EP physician has manually moved the EP catheter into place in the heart and is ready to begin robotic navigation. In Operational Mode, the Proteus™ II is moved into position by a technician or nurse, and the robotic arm is placed near the catheter entry point.
The catheter handle can then be installed and secured in the robotic arm’s Gripper at which point the robot goes into COBOT Mode. In COBOT Mode, which stands for collaborative robot, the Proteus™ II’s base is locked into a stationary position and the robotic arm can now be controlled by the EP surgeon via a Haptic controller for the fine-grain movements needed for the EP mapping and ablation procedures.
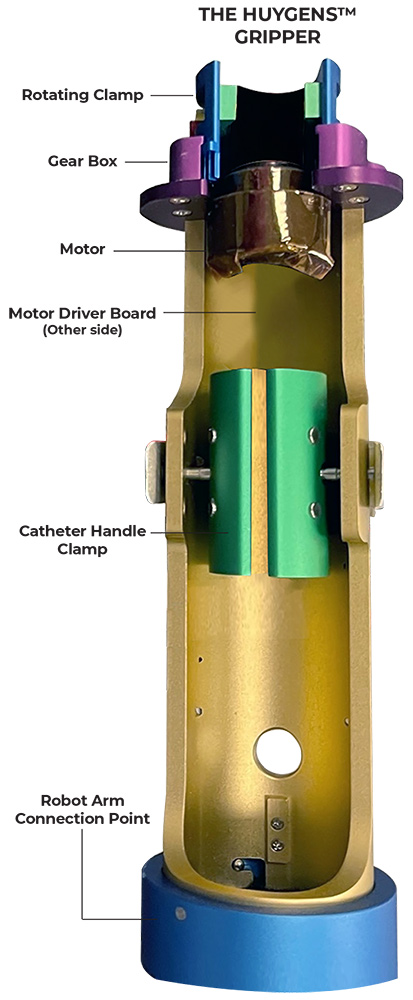
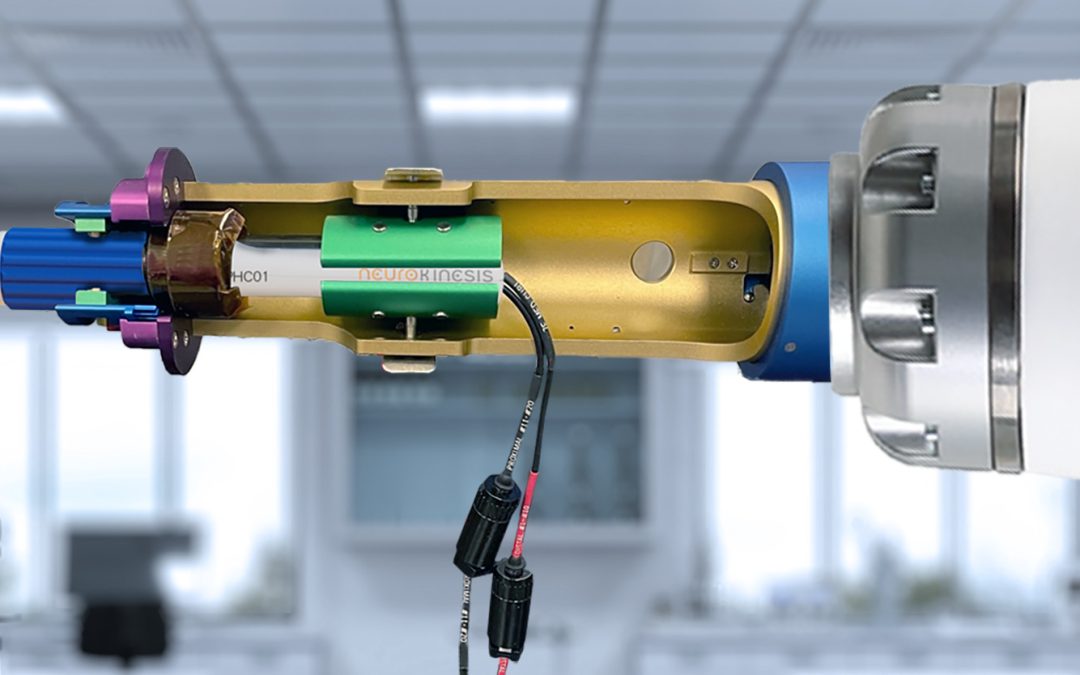
Catheter Handle Gripper
NKC was able to finalize its initial design for the Proteus™ II Gripper which is attached at the working end of the robotic arm, and to which a steerable catheter handle is secured for robotic navigation. The Gripper consists of a 254mm (10”) long, 50mm (2”) diameter catheter handle housing which has a connector plate that attaches it to the robotic arm. A motor-driver board controls a gear-driven rotating clamp which provides the deflection angle control of the catheter tip. The steerable catheter handle is secured in place by a tension clamp that allows for the use of any standard steerable catheter which uses a movable ring for deflection control. A control cable connects the Gripper’s control board to the system’s navigation control, and deflection is done by the EP physician turning a ring on the Haptic controller. With the Gripper in place, the Proteus™ II can manipulate a catheter in the three axes of direction needed for heart mapping and ablation: translation, rotation, and deflection.
Manufacture of the finalized prototype design of the Proteus™ II Gripper was awarded to RJC Manufacturing in Taiwan. RJC is a contract machine company that specializes in custom CNC machining and the turning of precision parts. RJC has more than 30 years of experience in manufacturing parts for the Defense Department, the Nuclear Energy sector, Medical Diagnostic Device developers, and many other industries.
The prototype of the Proteus™ II Gripper was received by the engineering team in December and was assembled onto the robotic arm for initial testing. For the test, the team used an industry-standard Live Wire Catheter with a steerable sheath. The catheter handle was secured into the Gripper and directional control tests were performed. For both gross movement and fine-grain control of the catheter for rotation and translation, the Gripper performed flawlessly. However, during deflection testing, the team found that excessive torque buildup was occurring between the deflection ring and the clamp when the tip was moved beyond 15O of angle. This caused the handle to slip in the housing. The team is currently making refinements to the design and anticipates having the revisions done by mid-January of next year at which point RJC will produce a new prototype.
With the Gripper, the Proteus™ II Robotic Arm’s mechanical and general navigation requirements will be complete. The focus now will be on the continued refinement of the navigation software and the development of the communications protocols which will allow the robotic arm to incorporate seamless manual control as well as AI to respond to data coming in real-time from the Huygens™ Catheter, the EnSite mapping system and the system’s Programmable Logic Controller (PLC). This ability will allow the Proteus™ II to be able to enhance both Human-In-The-Loop navigation and ultimately autonomous catheter navigation.
HUYGENS™ CATHETER
Development of NKC’s lead product candidate, the Huygens™ Catheter, saw major milestones met and exceeded this year. The Huygens™ Catheter will be the first EP catheter capable of performing both biopotential (topographical) mapping and bioimpedance (substrate) mapping of the human heart at the same time. In addition, the Huygens™ Catheter will be able to capture clinically significant data of bioelectric activity at a far lower microvolt level than the current art allows. In providing complete biopotential and bioimpedance measurements of an EP patient’s heart tissue down to a level of 25µV (microvolts) with high fidelity resolution, NKC hopes to provide EP physicians a level of diagnostic mapping that can significantly expand the ability to not just see the symptomatic results of arrhythmic activity but to understand the root cause. If successful, the Huygens™ Catheter technology will have a pivotal role in reshaping therapeutic cures for hundreds of thousands of patients (See “The Vision” below).
At the beginning of 2023, the NKC engineering team finalized the initial design of the catheter to achieve the stated engineering goals as follows:
- To develop an electrode sensor array that could capture bioimpedance measurements at or below the current state of the art.
- To develop a sensor array that could distinguish between near-field bioconductivity data and far-field interference such as blood-pool signals.
- To develop a signal-to-noise filtering process that would reduce, if not eliminate unwanted electrical noise from the desired bioimpedance signals.
- To develop an ability to convert the captured analog signals to incorruptible digital signals that could be transmitted to a mapping computer without any signal degradation or contamination.
- To develop a method to improve the representation of the energy contents on the spatial and time domains of the complex bioelectrical waveform.
- To develop a method to consistently ensure the relationship between the graphical representation and the underlying biopotential substrate is both predictable and accurate.
- To take all the above innovations, and use emerging micro miniaturization methods, put the entire bioelectrical signal capture, processing, and communication transmission onto a single flexible circuit board (FCB) embedded in the proximal end of the EP mapping catheter.
The first six goals would prove to be an engineering feat that on its own could advance the art. The achievement of the seventh goal would put NKC in a development space no one had ever succeeded in.
With the goals in place, the team had built the first bench-top test board to emulate the circuitry and processing capabilities that would eventually be put on the FCB of the Huygens™ Catheter. Through rigorous testing and revisioning of the components and firmware, the team was able to show that the concept for the catheter had been proven. In the second quarter of 2023, the Huygens™ test board was given to Sandia National Labs to perform the initial independent signal capture tests. Sandia was able to certify that the Huygens™ technology was able to accurately capture and display a variety of control-generated electrical waveforms with the same accuracy as current industry standard catheters.

The HUYGENS™ DIFFERENCE
The vision for the Huygens™ Catheter is not just to build a better mousetrap for diagnostic electrophysiology, but to completely reimagine what is possible for the art. Currently, there are close to 20 major manufacturers who make more than 1,000 different types of diagnostic catheters for the EP field. Yet none of them have the differentiators that make the Huygens™ Catheter not only a wholly unique product but one that has to ability to change the possibilities for EP therapeutic cure.
OURS
- All data acquisition and processing occurs at the point of capture.
- Can capture clinically relevant bioelectric signals as low as 25 µV.
- Can perform both biopotential and bioimpedance capture at the same time.
- New half-round electrode pairing greatly reduces signal contamination from far-field noise sources.
- All processed data is converted to an incorruptible digital signal before being sent to mapping/PLC stations.
THEIRS
- All data acquisition and processing occurs up to 20’ away at an external mapping station.
- Can only capture clinically relevant signals down to 100 µV or higher.
- Can only capture biopotential or bioimpedance signals during mapping.
- Concentric rings are unable to differentiate near-field and far-field signals.
- All signal capture is analog from the electrode to the mapping station and is subject to noise contamination and degradation.
In addition to this important third-party certification, the engineering team has been able to advance progress on the Huygens™ Catheter on several fronts including:
- Improvements in the signal-to-noise filtering and amplification to allow lower microvolt readings (=<100 µV) to be captured and displayed.
- The ability to provide automatic switching between bioimpedance and biopotential signal capture to be performed sequentially during a single pass with a minimal signal loss between switching that does not compromise clinically relevant data capture needs.
- An ability to allow the EP physician to manually switch from auto-capture to biopotential only or bio-impedance testing only.
- Refinements to the digital-to-analog converter (DAC) processing to enable faster and noise-protected data processing to the EnSite mapping system.
- Modifications to the catheter guide-wire mechanism that controls catheter tip deflection.
- Developed the ability to connect the FCB to the test board for firmware updating and electrical testing.
- Development of the Catheter Handle PCB Control Board which provides the interface between the FCB and the outside power and communications systems.
- Finalized and installed the communication and power port interfaces for the connecting cables between the catheter, the PLC, and the Ensite mapping system.
- Finalized the design of the Huygens™ Catheter shaft needs, and developed the manufacturing specs for such.
- Have performed multiple tests of the ability of the catheter to capture, measure, and display simulated QRS signals in preparation for the next series of Sandia certifications.
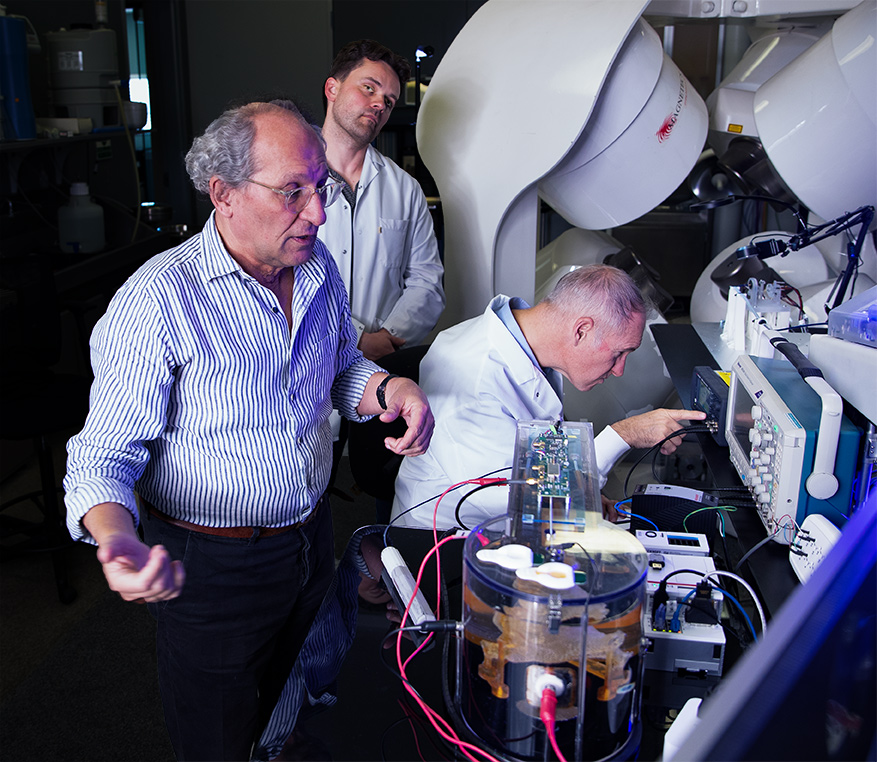



The NKC Engineering Team consists of experienced professionals with expertise in all the disciplines needed to bring NKC’s vision for its Huygens™/Proteus™ technology to market. Pictured above (clockwise from top left): CTO and co-founder Josh Shachar observes simulated QRS testing of the Huygens™ Catheter, an NKC engineer tests the prototype of the Huygens™ Catheter handle for manual navigation inside the wet-lab heart model, An NKC software/firmware engineer reviews the FCB schematics for the Huygens™ Catheter. An NKC electro-mechanical engineer tests the communication between the Huygens™ Catheter and the EnSite Navigation system.
All this work was done in anticipation of being ready for the planned upcoming animal study at the Technion Institute in Haifa, Israel at the beginning of the new year. Unfortunately, NKC was faced with three unanticipated challenges which required an adjustment to the timelines and a rethinking of certain strategies.
The first was an observation by the engineering team when the latest firmware update was done to the FCB. The firmware update included several new upgrades to the functionality of the FCB for performance and functionality. Currently, the FCB is updated and powered by a connection to the test board. With the latest update though, the FCB was not able to effectively run the new system. The engineering team believes this is due to a power incompatibility between the test board output and the FCB requirements. Indeed, it is possible that this problem is not a problem at all as the observation is of the FCB being powered independently and not in series with the catheter handle control board which is the intended configuration. The team has already postulated the possible issues and has developed solutions to eliminate the problem. This will be done in the first week of January when the team returns from the holiday break.
The second problem was not wholly unanticipated and one that is common to all enterprises who are seeking to develop strategic partner relationships for both short-term and long-term needs. NKC had been in discussion with SEISA Medical to provide manufacturing for the first prototypes of the Huygens™ Catheter to be used for both in-house testing and the animal study, with an eye to becoming NKC’s partner for scaled manufacturing of the catheter in the future. SEISA Medical is a global full-service contract manufacturer of Class II and Class III medical devices with over 350,000 square feet of manufacturing space and 150,000 square feet of Class 7 and 8 cleanroom capacity around the globe. Though only providing prototype services currently SEISA is capable of expanding services to include 510K generation services and vertically integrated device manufacturing capabilities.
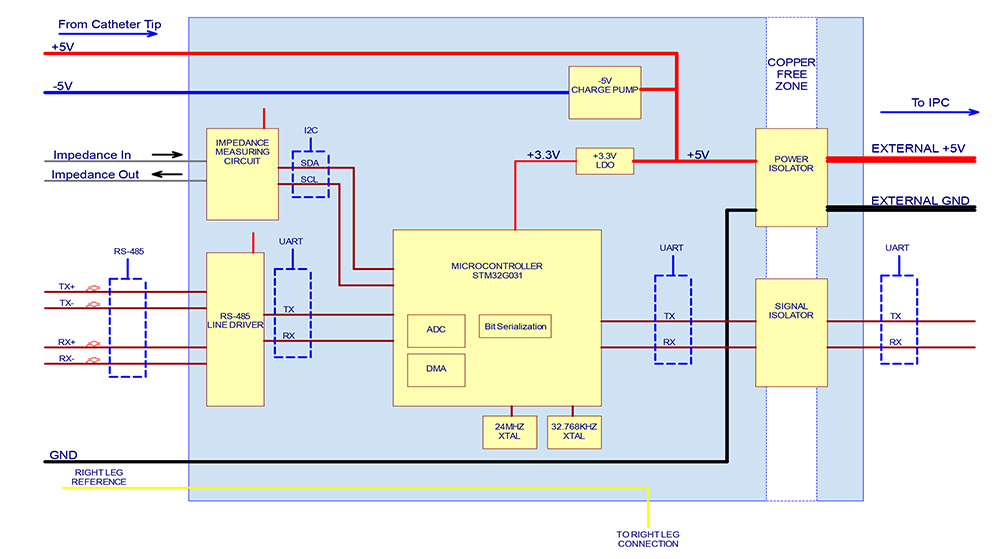
The diagram on the left shows the power-flow, communication control, and signal processing layout for the Huygens™ Catheter Handle control board (shown on the right) which provides all data and electrical system management between the PLC, the navigation station, the mapping station, and the Huygens™ FCB.
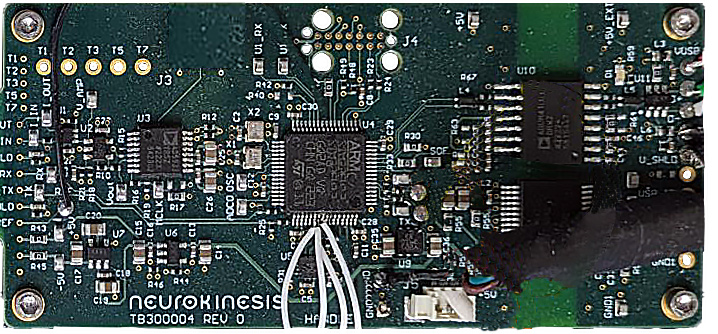
NKC released the initial design specification for the Huygens™ Catheter to SEISA back in November. As with most manufacturing quotes in this post-pandemic world, the estimated cost and lead times for delivery greatly exceeded NKC’s projections. To ensure its fiduciary responsibility to its investors and the current budgets, the NKC management team decided to solicit RFQs from several other leading catheter manufacturers to try and leverage the immediate needs and potential long-term requirements. NKC expects to have all the quotes in hand in the first week of January at which time they will review cost and abilities. During this time, NKC will begin fabrication of the Huygens™ Catheter Handle control boards and its partner Sierra Circuits will produce the latest version of the FCBs. Sierra is a 60-year-old company that specializes in printed circuit board (PCB) manufacturing. Their facility houses over $45 million dollars in PCB design, fabrication, and testing infrastructure and they hold 15 patents for their proprietary manufacturing processes.
Assuming that an agreement can be made between NKC and a catheter vendor and that the issue with the FCB is resolved, NKC hopes to deliver the final specs and parts to the selected manufacturer no later than the end of January with an eye for delivery by no later than the end of the first quarter of 2024.
The final challenge was wholly unexpected. The conflict that occurred on October 7th in Israel has resulted in the shutdown of any research studies at the Technion Institute until the war is resolved. In light of this development, NKC has decided to shift the initial study from Israel to Torrence, California.
ANIMAL STUDY
Due to the ongoing hostilities in Israel, and with a desire to not further delay the planned animal study, the NKC management team in consultation with the study’s director, Dr. Eli Gang, has decided to move the work over to the Harbor-UCLA Medical Facility in Torrence, California. The Harbor-UCLA Medical Facility houses a dedicated 20,000-square-foot research lab for studies such as the one NKC has proposed. The lab includes tissue culture facilities, isotope stabilization capabilities, molecular biology analysis tools, morphology equipment including electron and confocal microscopy, a PALM micro-dissection system, a modern animal surgical suite, and vivarium, and all other instrumentation for state-of-the-art endocrine and metabolic research.
Because of this change, the science team in conjunction with NKC’s regulatory affairs consultant Professor Elaine Duncan, is working on adapting the previously written protocols to accommodate the requirements for ensuring the validity of the study’s objectives, process, and the resulting data that will be acquired both for ongoing research and development, but also for FDA review as the company seeks approval for a Humanitarian Use Device (HUD) with a Humanitarian Device Exemption (HDE). The HUD and HDE will allow the company to contemplate doing its first human clinical trials addressing patients with arrhythmia issues related to low-voltage scarring in the heart. The company also hopes the data will be sufficient for submission for CE Mark approval in the EU.



The Harbor-UCLA Medical Facility offers a state-of-the-art animal research lab connected to one of the area’s most comprehensive medical facilities. Harbor-UCLA has a proven reputation for meeting all the criteria that researchers, physicians, and regulatory agencies look for when determining the foundational needs for performing trustworthy investigatory studies.
To review, the animal study will be looking to gather data in two areas, GLP and non-GLP. For the GLP (Good Laboratory Practice) portion of the study, NKC will be acquiring data related to the catheter’s overall ability to meet the criteria for a Class II medical device. For FDA purposes, NKC will need to show that the Huygens™ Catheter demonstrates “reasonable assurance of the safety and effectiveness of the device” as it relates to their 21 CFR Part 861 requirement. In addition, the FDA requires that all medical Class II device manufacturers establish mission-critical processes and procedures to show the device meets all applicable laws and regulations. The 21 CFR Part 820 requirement outlines the unique methods, controls, and systems for “designing, purchasing, manufacturing, packaging, storing, and installing and servicing medical devices.”
For the non-GLP portion of the study, the NKC team will be gathering data on all the various functions of the Huygens™ Catheter. This includes:
- The ability of the catheter to integrate with the EnSite mapping system to produce an accurate heart map.
- The ability of the catheter to capture, process, and transmit bioimpedance data.
- The ability of the catheter to capture, process, and transmit biopotential (substrate mapping) data.
- The ability of the catheter to target and acquire clinically relevant data of electro-anatomical signals at the pre-defined areas of both the left and right atrium and the left and right ventricle of the test subject’s heart.
- The ability of the catheter to repeatably reach preselected anatomically significant targets.
- Data to allow comparison of the catheter’s signal fidelity in comparison with the existing art.
- Data related to procedure time, measurements of stimulation thresholds at selected anatomic sites, analysis of surface and intracardiac signal recordings during target acquisition, and postmortem effects on the test subject’s heart.
Each individual aspect of the study will be conducted in isolation of the others to ensure there is no conflict of variable concerns when the resulting data is analyzed and submitted to the FDA. With this shift in the logistics for the animal study, NKC now believes it will begin the study during the second quarter of 2024. The in-lab portion of the study is anticipated to only take 2-3 days to complete. Subsequent analysis of the data from the study, preparation of documents for submission to the FDA, and any adjustments to the R&D timeline should be completed in the 30- to 60-day window after the completion of the study.
WHAT IS A GLP STUDY?
A Good Laboratory Practice (GLP) study refers to research or testing study conducted in accordance with established GLP principles and regulations. GLP is a quality system that ensures the consistency, reliability, and integrity of non-clinical laboratory studies, particularly those conducted for regulatory purposes, such as assessing the safety of chemicals or pharmaceuticals.
Key features of a GLP study include:
- Rigorous documentation is a fundamental aspect of GLP. Detailed records of study protocols, procedures, raw data, and results are maintained to ensure traceability and accountability.
- Incorporation of a robust quality assurance program. This involves systematic and independent inspections and audits to verify compliance with GLP principles and regulations.
- GLP studies follow standardized and well-documented operating procedures to ensure consistency and reproducibility of results. These procedures cover various aspects of the study, including sample handling, analytical methods, and data recording.
- Personnel involved are adequately trained for their respective roles. Training records are maintained to demonstrate that individuals conducting the study are qualified and competent.
- Studies are conducted in facilities that meet specific standards. This includes appropriate laboratory space, equipment calibration and maintenance, and environmental controls to ensure the reliability of study results.
- A detailed study protocol is developed before the initiation of the study. The protocol outlines the objectives, study design, methodologies, and acceptance criteria for the study. Any deviations from the protocol are documented and explained.
- All study-related documentation, including raw data, final reports, and samples, is archived in a secure manner for a specified period. This archival process is critical for traceability and the ability to reconstruct the study if necessary.
- A compliance statement is included in the final report. This statement confirms that the study was conducted in compliance with all the above GLP principles.
GLP standards are typically enforced by regulatory agencies, and studies conducted following GLP principles are often submitted to regulatory authorities as part of applications for product approvals or registrations. Compliance with GLP is important for ensuring the reliability and acceptance of non-clinical safety data in the evaluation of the potential risks associated with chemicals, pharmaceuticals, medical devices, and other products.
THE VISION

NKC Co-Founders Josh Shachar and Dr. Eli Gang take a moment to celebrate a milestone in their technology development work.
As 2023 draws to a close, it is a good time to re-share with our investor family the vision that has driven NKC from an idea to the reality of where we stand today. The dream of developing a better tool for navigation came like a lot of great ideas, not from a team of engineers pounding away at whiteboards trying to think of a new invention, but rather from a personal tragedy founder and CTO Josh Shachar experienced back in 1993 when Josh’s mother underwent a catheter stent operation. Josh was allowed to watch and as he observed the physician trying to navigate a three-foot-long wire into and around his mother’s heart, he thought that there had to be a better way to do this with far greater control and accuracy that was not wholly dependent upon all the variables that can affect human performance.
As a result, Magnetecs was born which resulted in the creation of the Catheter Guidance Control and Imaging (CGCI) system. CGCI was the first robotic-assisted magnetically controlled navigation system for the guidance of an EP catheter inside the living environs of a human heart. The system was revolutionary in many ways and achieved several critical benchmarks and milestones during its development. Along the way, Josh and his team discovered that the need for better catheter navigation was seconded only by the need for a better mapping catheter.
As Josh became more enmeshed in the science of EP, he discovered that in many ways EP medicine was more of an art form that relied upon the ability of the EP physician’s experience to take the limited data that EP mapping catheters provided and to then make therapeutic decisions about how to approach an ablation procedure to address arrhythmia issues such as AFib, or tachycardia. At the time, mapping catheters only delivered reliable data on the bioimpedance (topography) of the myocardial tissue at levels of 100 µV and above. Although such readings and the resulting maps were generally useful in 80% percent of the cases where the electrical abnormalities were so large that simply addressing those issues in the ablation procedure was sufficient to generally restore rhythmic health to the heart muscle, for the remaining 20%, and for a good number of the 80% who suffered secondary arrhythmia issues, the current mapping data was not able to “tell the story” of what was happening symptomatically below the 100 µV level, let alone what the actual root cause of the issues was.
It was then in 2009 that Josh and the team began to work on a new generation of mapping catheters called MOSFET (Metal-Oxide Semiconductor Field-Effect Transistor). Though currently several companies have begun work using core principles of the MOSFET catheter, at the time Josh was once again way ahead of the science. His concept was to take all the data processing of the captured bioelectric signals from the catheter electrodes and put them onto a circuit board embedded in the catheter tip. At the time mapping catheters worked by electrodes in the catheter tip capturing analog bioelectric signals that then had to travel up to six meters along the catheter sheath to a processing computer and then to a mapping system in the operating room. Along the way, the signal was subject to many sources of noise contamination and signal distortion, which made it nearly impossible to “see” any clinically relevant data that lay in the lower voltage spectrums. By using micro-miniaturization techniques, Josh envisioned being able to allow the captured signals to be filtered and amplified by the circuit board in the catheter tip before being sent to the mapping catheter.
WHAT
Lies
BENEATH THE SURFACE
The efficacy of therapeutic cure in electrophysiology is entirely dependent upon accurate diagnostic determinations. In restoring long-term stability for the patient’s heart to produce pacing rhythms that meet the demands of the rest of the body for oxygenation, nutrient delivery, and waste removal that the circulatory system provides, the EP physician must have a reliable picture of all the parameters that might be contributing to poor sinus rhythms. Critical to that data is an accurate map of how electrical signals are flowing, where they are being disrupted, and why those disruptions are happening. If an EP physician has a complete picture that describes those questions, then they are better able to make determinations on how an ablation process can restore health to a heart beating out of sync.
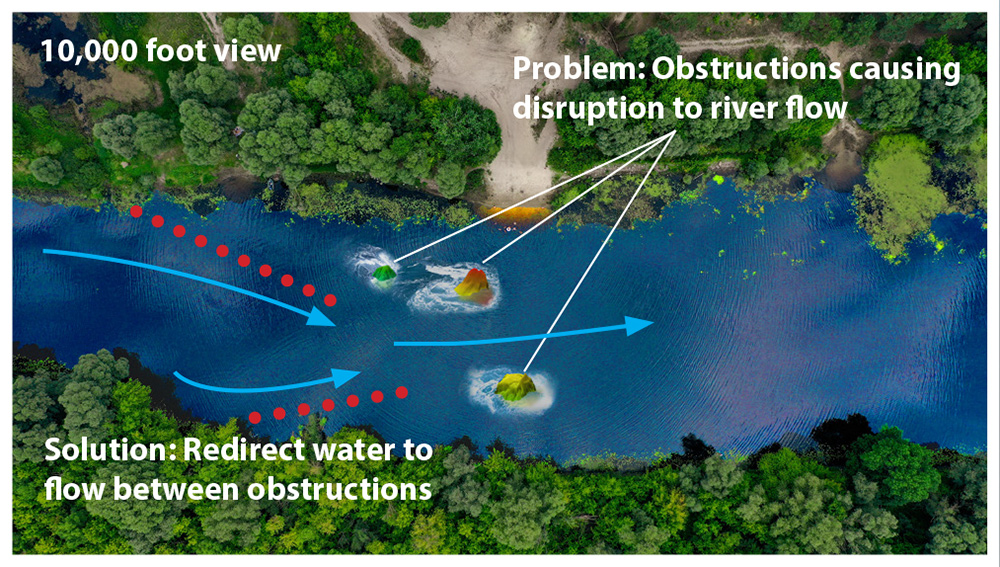
The EP heart map provides the physician with the road-map from which they make decisions on how to redirect electrical flow to restore abnormal pacing issues. Most EP mapping procedures only involve capturing bioimpedance information to provide the equivalent of a topographical map of the heart which only gives indications of where electrical flow is being disrupted on the surface.
The image on the right illustrates this by showing a picture of a section of a river from 10,000 feet. At this level, the snapshot shows how three large rocks are interrupting the water’s flow. Based solely on this information, the best corrective action would be to build a dam upriver from these obstructions that would channel the water in between the two large rock formations to maximize the flow to the center of the river.
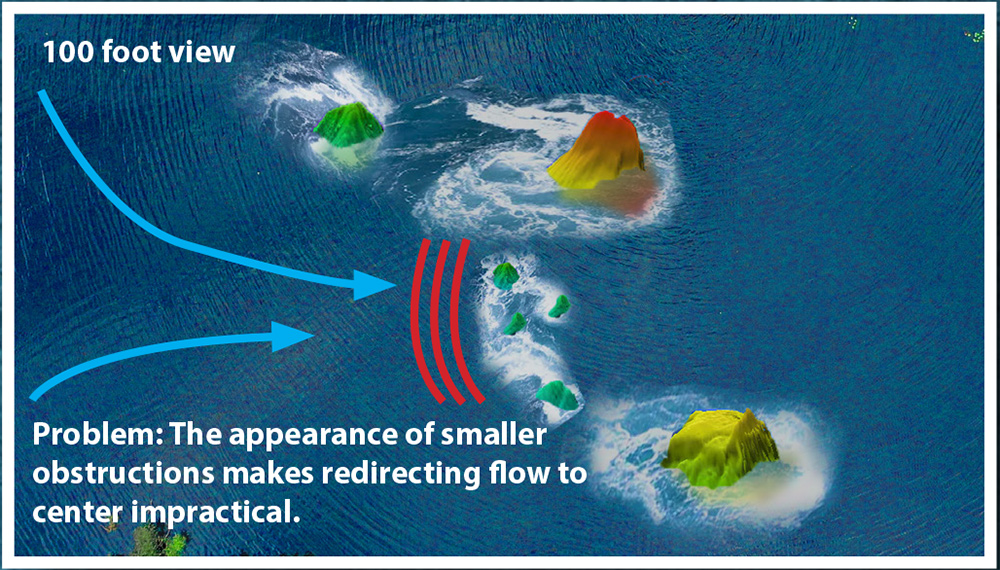
What this view of the river is not able to show is that when you get closer to the surface and take a picture, the visibility of smaller obstructions is easy to see. As these become visible it also becomes apparent that directing the water between the two large formations would create a larger disturbance to the river’s flow.
Further, neither of these surface-level images shows what is happening below the waterline. As can be seen, the obstructions on the surface are only the “tip of the iceberg” to what is causing the flow issue. In EP heart mapping, being able to see this substrate can make all the difference between an ablation plan that will work and one that won’t. The Huygens™ Catheter provides the ability to not only see the small obstructions (scar tissue) that can cause disruptions but also the quality of the underlying tissue.
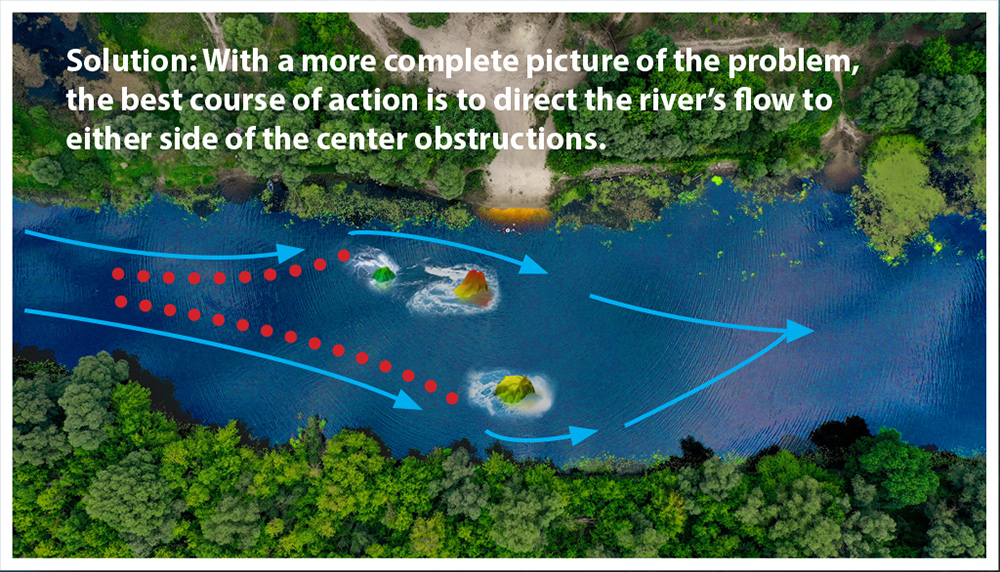
By providing both bioimpedance (topographical) and biopotential (substrate) mapping at the same time, The EP physician has a much more comprehensive picture to decide how to redirect electrical signals. Using our illustration of the river, the best course of action would be to direct the water to either side of the center where there are no surface or underlying obstructions to interrupt the water’s flow.

“There is today a whole community of scientists and physicians that are waiting for the solution we believe the Huygens™ Catheter can deliver.” – Josh Shachar
In a recent conversation with Josh Shachar regarding the need for a technology like Huygens™’ he stated, “The nexus between the art of medicine and the discipline of scientific discovery has always been that juncture where the ability to provide better information about the “what is” gives the physician a better picture of the “why” a patient is having an issue which then allows them to make a better decision on the “how to” in their therapeutic care. In diagnostic electrophysiology, the current standard only allows a physician to see the effects of the electrical disturbance that is happening on the surface of the heart. To the extent that sometimes current substrate mapping can also help a physician see under the surface tissue, for the most part, the art of EP continues to work with very incomplete pictures of what is actually happening when a patient has a non-sinus rhythm condition. The whole vision of the Huygens™ Catheter is to not just get a better picture of the endocardial tissue but to be able to see all the way through the heart muscle to get a clear picture of what is going on under the surface, at the myocardial, epicardial and pericardial levels. If we can do that, we open the window of the physician to not just see what is happening, but why it is happening. The therapeutic decisions that can be made from that perspective propose a whole new level of cure for EP patients around the world.”
To use an analogy we have used previously, imagine flying over a large river at 20K feet. At that level, one might be able to see where large objects such as rocks might be creating disturbances on the surface of the water thereby disrupting flow. If your job is to minimize the impact of this disturbance you would think the solution would be to build a dam upriver from the rock that would gently divert the water around the tip of the rock you saw. What you would not know from your perspective though is that there were lots of smaller rocks around the large rock you could not see that were impacting the current, let alone the fact that the tip of the large rock was part of a very large obstruction that went all the way to the bottom and halfway across the river bed so that simply correcting the flow of water at the surface, did nothing to correct or actually increased the flow of current below the surface.
It is this vision to expand the information the EP physician has, to allow them to use their healing artistry for better results that drives NKC’s efforts. As Josh has stated, “There a literally thousands of articles and research papers in the field today discussing what scientists and doctors believe about the impact of micro-volt level disturbances and small scar tissue formations that are creating issues with the pacing of the heart. We can see the effects and we know something is causing it, we just have not been able to quantitatively measure these issues. In some ways, it is like the discovery of gravity. Geniuses like Galileo who wondered why objects move, DaVinci, who worked to describe what we know as gravitational constants, or Nicolaus Copernicus who created the heliocentric model. All of them and many others knew that something was behind all this but it took Isaac Newton to finally do the math and give it a name before science was able to make the tremendous leaps forward that we have seen that have resulted in the myriad of technologies we take for granted today that applied that discovery.
“As then, there is today a whole community of scientists and physicians that are waiting for the solution we believe the Huygens™ Catheter can deliver. And when we deliver, the world of Electrophysiology will never be the same.”
A WORLD IN NEED
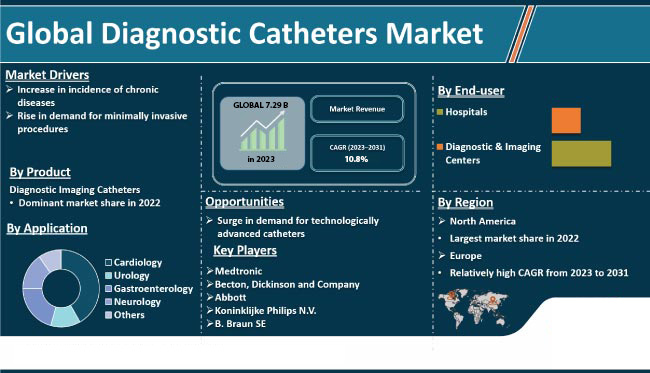
Electrophysiology continues to be an ever-expanding field of science and healthcare. In 2017 the global market for electrophysiology procedures was valued at $5.10B and $7.29B in 2023 of which half of that was for cardio-ablation procedures. By 2027 it is expected to reach a value of $11.38B with a CAGR of 10.8% .* In 2012 approximately 2.2 million people were diagnosed with AFib. That number is expected to increase to more than 12 million people by 2030 as issues such as increasing population, hypertension, diabetes, obesity, and many other factors related to increased health factors that can impact cardiovascular health, continue to rise. In treating these issues, the American Heart Association has stated that the limited efficacy of anti-arrhythmic drug therapy for the treatment of AFib continues to drive a demand for ablation procedures which have a far higher success rate. In 2016, catheter-based EP procedures surpassed that of cardiac implantable electronic device procedures, and most analysts anticipate a CAGR growth rate of 23.9%. In 2020, over 360,000 ablation procedures were performed in the US alone. The current device cost per ablation procedure averages about $10,500 with $1,750 of that for the mapping catheter alone. For the US, this represents a more than a half-a-billion dollar market sector.
As these numbers show, the need for, and the market demand for the technology that NKC is providing continues to grow. The value that NKC can bring to this demographic underscores the generous investment of time and money that management, engineering, and most importantly our investment family has and continues to make.
SAFE HARBOR DECLARATION
THE STATEMENTS, PROJECTIONS AND ESTIMATES OF FUTURE PERFORMANCE OF THE COMPANY OR VARIOUS ELEMENTS OF THE COMPANY’S BUSINESS CONTAINED IN THIS MEMORANDUM THAT ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD EXPECT THAT ANTICIPATED EVENTS AND CIRCUMSTANCES MAY NOT OCCUR, THAT UNANTICIPATED EVENTS AND CIRCUMSTANCES WILL OCCUR, AND THAT ACTUAL RESULTS WILL LIKELY VARY FROM THE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD BE AWARE THAT A NUMBER OF FACTORS COULD CAUSE THE FORWARD-LOOKING STATEMENTS OR PROJECTIONS CONTAINED IN THIS MEMORANDUM OR OTHERWISE MADE BY OR ON BEHALF OF THE COMPANY TO BE INCORRECT OR TO DIFFER MATERIALLY FROM ACTUAL RESULTS. SUCH FACTORS MAY INCLUDE, WITHOUT LIMITATION, (i) THE ABILITY OF THE COMPANY TO COMPLETE THE DEVELOPMENT OF ITS PRODUCTS IN A TIMELY MANNER, (ii) THE DEMAND FOR AND TIMING OF DEMAND FOR SUCH PRODUCTS, (iii) COMPETITION FROM OTHER PRODUCTS AND COMPANIES, (iv) THE RESULTS OF THE COMPANY’S SAFETY AND EFFICACY STUDIES, (v) THE RESULTS OF THE REGULATORY APPROVAL PROCESS, (vi) THE COMPANY’S SALES AND MARKETING CAPABILITIES, (vii) THE COMPANY’S ABILITY TO SELL ITS PRODUCTS PROFITABLY, (viii) THE ABILITY OF THE COMPANY’S THIRD-PARTY SUPPLIERS TO PROVIDE PRODUCTS AND SERVICES IN A RELIABLE MANNER; (ix) AVAILABILITY OF ADEQUATE DEBT AND EQUITY FINANCING, AND (x) GENERAL BUSINESS AND ECONOMIC CONDITIONS. THESE IMPORTANT FACTORS AND CERTAIN OTHER FACTORS THAT MIGHT AFFECT THE COMPANY’S FINANCIAL AND BUSINESS RESULTS ARE DISCUSSED IN THIS MEMORANDUM UNDER “RISK FACTORS.” THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL BE ABLE TO ANTICIPATE, RESPOND TO OR ADAPT TO CHANGES IN ANY FACTORS AFFECTING THE COMPANY’S BUSINESS AND FINANCIAL RESULTS.